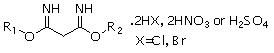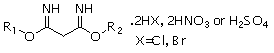Derivatization gas chromatography for rapidly measuring dialkoxy propyl-diamidine salt
A dialkoxy propane diamidine salt and gas chromatography technology is applied in the field of derivative gas chromatography for rapid determination of dialkoxy propane diamidine salts, and can solve the problem of lack of detection ability, strong pertinence and lack of versatility and other problems to achieve the effect of overcoming poor universality and enriching analytical methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Derivatization pretreatment of samples
[0025] Weigh ~0.2g of the sample to be tested into a 10ml sample tube, add 2.0g of derivatization reagent-1 [mixture of dilute phosphoric acid (5% by weight) and methanol (25% V / V)], shake to dissolve, and ultrasonically hydrolyze for 20 Minutes, dry with ~2g of anhydrous sodium sulfate, let stand for 5 minutes, centrifuge to get the supernatant.
[0026] (2) Setting of instruments and testing conditions
[0027] Instrument: Shimadzu GC-2010 Plus;
[0028] Chromatographic conditions: chromatographic column: PEG-20000 (30m×0.32mm×0.25μm) capillary column; inlet temperature: 200°C; FID detector temperature: 250°C; carrier gas: high-purity nitrogen, flow rate 2.0ml / min; Hydrogen: 35ml / min; Air: 450ml / min; Injection method: Split injection, split ratio: 1:30; Makeup gas flow: 20ml / min; Injection volume: 2.0μl; (No hold), raise the temperature to 280°C at 20°C / min, and hold for 10 minutes.
[0029] (3) Actual testing of sample...
Embodiment 2
[0036] (1) Derivatization pretreatment of samples
[0037] Weigh ~0.2g of the sample to be tested in a 10ml sample tube, add 2.0g of derivatization reagent-1 [mixture of dilute sulfuric acid (5% by weight) and methanol (25% V / V)], shake to dissolve, and ultrasonically hydrolyze for 20 Minutes, dry with ~2g of anhydrous sodium sulfate, let stand for 5 minutes, centrifuge to get the supernatant.
[0038] (2) Setting of instruments and testing conditions
[0039] Instrument: Shimadzu GC-2010 Plus;
[0040] Chromatographic conditions: chromatographic column: OV-17 (30m×0.32mm×0.50μm) capillary column; inlet temperature: 250°C; FID detector temperature: 250°C; carrier gas: high-purity nitrogen, flow rate 2.0ml / min; Hydrogen: 35ml / min; Air: 450ml / min; Injection method: Split injection, split ratio: 1:30; Makeup gas flow: 20ml / min; Injection volume: 2.0μl; (No hold), the temperature was raised to 250°C at 15°C / min, and held for 10 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com