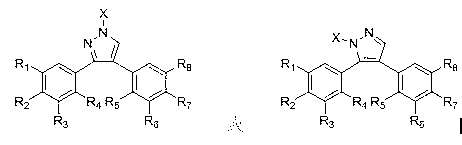
Diaryl pyrazole compound, and preparation method and purpose thereof
A technology for diarylpyrazoles and compounds, applied in the field of medicine, can solve the problems of insufficient activity, complex synthesis, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
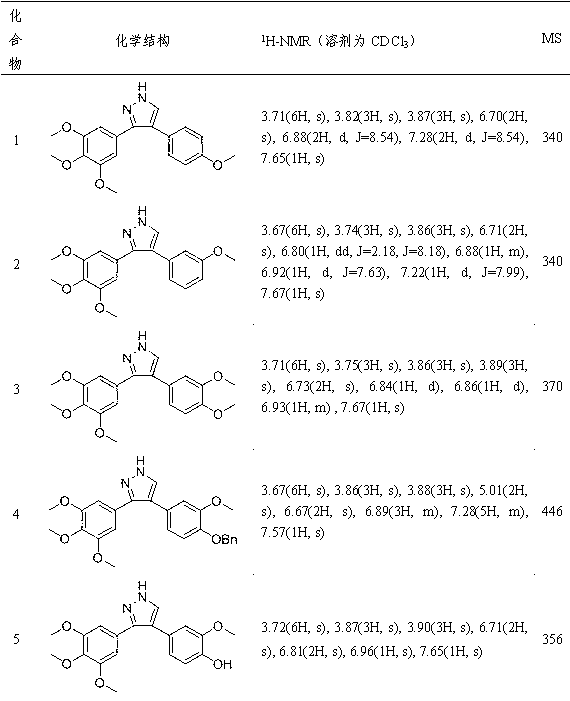
[0189] Example 1: Preparation of 3-(3,4,5-trimethoxyphenyl)-4-(4-methoxyphenyl)-pyrazole (compound 1)
[0190] Dissolve 3-(3,4,5-trimethoxyphenyl)-4-(4-methoxyphenyl)-2-methylene ethyl ketone in methanol, add 25 equivalents of triethylamine and 5 The equivalent of semicarbazide hydrochloride was refluxed for 6 hours. After the reaction is completed, add 2 equivalents of 20% sodium hydroxide aqueous solution, and continue to reflux for 6 hours. After the reaction, the methanol was evaporated under reduced pressure and then extracted with ethyl acetate. The organic layer was washed with saturated sodium chloride solution and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the compound 1 was separated and purified by column chromatography, with a yield of 65%; the structural formula of compound 1, 1 H-NMR and MS data are listed in Table-1 below.
Embodiment 2
[0191] Example 2: Preparation of 3-(3,4,5-trimethoxyphenyl)-4-(3-methoxyphenyl)-pyrazole (compound 2)
[0192] Except for using the corresponding raw materials, compound 2 was prepared by the same method as in Example 1, with a yield of 65.5%; the structural formula of compound 2, 1 H-NMR and MS data are listed in Table-1 below.
Embodiment 3
[0193] Example 3: Preparation of 3-(3,4,5-trimethoxyphenyl)-4-(3,4-dimethoxyphenyl)-pyrazole (Compound 3)
[0194] Except for using the corresponding raw materials, compound 3 was prepared by the same method as in Example 1, with a yield of 55.3%; the structural formula of compound 3, 1 H-NMR and MS data are listed in Table-1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com