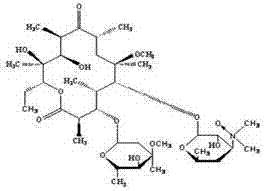
Preparation method of clarithromycin-N-oxide
A technology for clarithromycin and clarithromycin is applied in the field of preparation of clarithromycin-N-oxide, and achieves the effects of cost reduction, easily controllable conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Take 0.015 mol of clarithromycin raw material with a mass of 11.2 g, put it into a reaction bottle equipped with a condenser, add 500 mL of 50% acetonitrile solution, and stir at a temperature of 80-100 °C until clarithromycin dissolves;
[0041] (2) Add 40mL of 10% hydrogen peroxide solution to the reaction bottle under the condition of 50~60℃, react for 2 hours, add 80mL of 10% hydrogen peroxide solution, react for 2h, add 80mL of 10% hydrogen peroxide solution, and react for 2h, get the reaction solution;
[0042] (3) Evaporate the reaction solution to dryness in a water bath at 100°C, and remove residual H 2 o 2 11.4 g of the residue was obtained, and the residue was recrystallized with 50% acetonitrile as a solvent to obtain clarithromycin-N-oxide.
[0043] The steps of recrystallization are as follows:
[0044] (1) Crystallization: Add 100 times the weight of the residue to 50% acetonitrile, heat to 80°C, stir to dissolve the residue, filter, concentrate th...
Embodiment 2
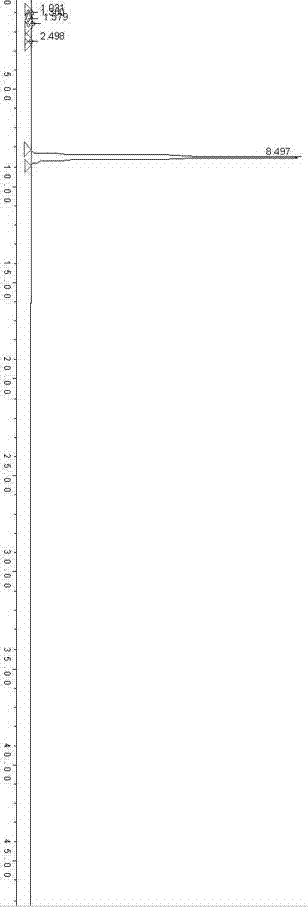
[0049] Compared with Example 1, the hydrogen peroxide solution with a mass concentration of 10% was added twice, 100 mL each time, and the rest of the operation steps were exactly the same as in Example 1. How many 9.4g crystals are obtained, and the productive rate is 82%. The HPLC method is used to detect the clarithromycin-N-oxide compound obtained by the method of the present invention. figure 2 . The purity of the clarithromycin-N-oxide obtained by the method of the present invention reached 95.8% using the peak area normalization method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com