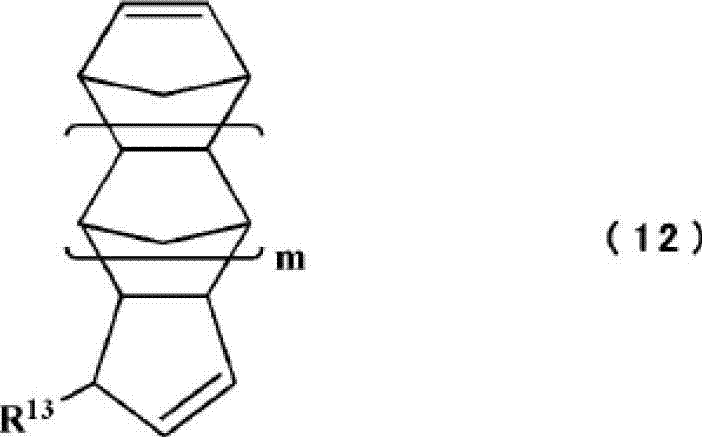
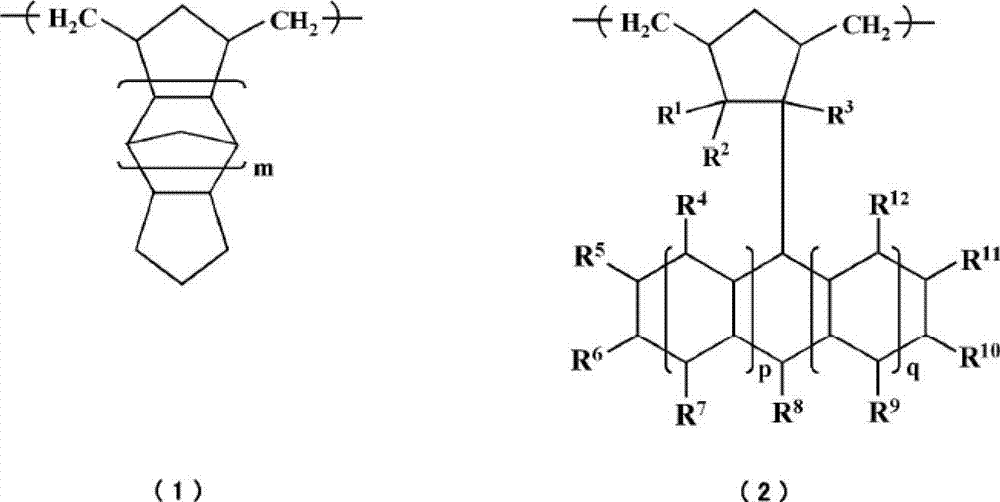
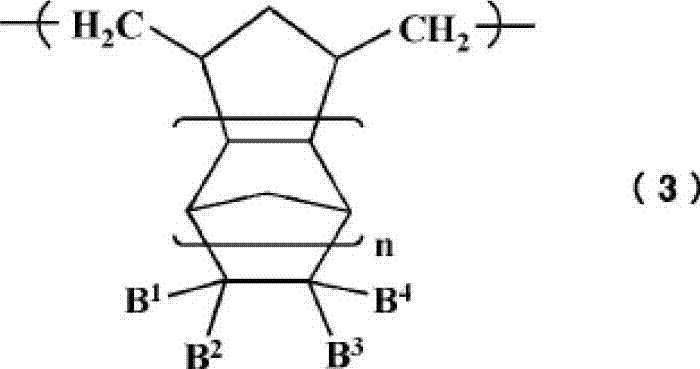
Cycloolefin-based ring-opening copolymer
A cycloolefin and copolymer technology, which is applied in the field of cycloolefin ring-opening copolymers, can solve the problems of unknown physical properties of ring-opening copolymers, and achieve low birefringence, low oxygen permeability, and excellent formability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0137] Hereinafter, the present invention will be described more specifically based on examples, but the present invention is not limited to these examples. In the following examples and comparative examples, various steps such as polymerization reaction and catalyst preparation were carried out under a nitrogen atmosphere.
[0138] In addition, each measurement and evaluation can be performed by the following methods.
[0139] Glass transition temperature (Tg):
[0140] Using a differential scanning calorimeter (manufactured by Seiko Instruments, trade name: DSC6200), the extrapolated glass transition initiation temperature was defined as the glass transition temperature (Tg) in accordance with Japanese Industrial Standard K7121.
[0141] Weight average molecular weight and molecular weight distribution:
[0142] Using gel permeation chromatography (GPC, manufactured by Tosoh Corporation, trade name HLC-8020), using tetrahydrofuran (THF) as a solvent, the weight average mol...
example 1
[0151] 5-phenyl-bicyclo[2.2.1]hept-2-ene (“phenylnorbornene” (represented as Ph-NB in Table 1)) (13.5 g, 132 mmol ), Wuhuan [6.5.1.0 2,7 .1 3,6 .0 9,13 ]pentadeca-4,10-diene ("tricyclopentadiene" (indicated by TCP in Table 1)) (46.5g, 391mmol), 1-butene (0.37g, 6.59 mmol) was added to cyclohexane (107 g) and methylcyclohexane (19 g), and it heated and stirred at 100 degreeC. Separately, a solution obtained by adding a ruthenium carbene complex catalyst (0.684 mg, 1.046 μmol) represented by the following formula (11) to toluene (0.55 g) was prepared. To the aforementioned monomer solution, the ruthenium carbene complex catalyst solution was added to start the polymerization reaction. After 1 hour of polymerization, a toluene (0.55 g) solution of ethyl vinyl ether (0.0735 g, 1.046 μmol) was added as a reaction stopper to obtain a ring-opened copolymer solution. The conversion rate of the monomer was measured and found to be 99% by mass. A ring-opened copolymer was obtain...
example 2
[0157] Phenylnorbornene (expressed as Ph-NB in Table 1) (18 g, 176 mmol), tricyclopentadiene (TCP) (42 g, 353 mmol) as a cycloolefin monomer, and as a molecular weight regulator The 1-butene (0.37g, 6.67mmol) of a reagent was added to cyclohexane (107g) and methylcyclohexane (19g), and it heated at 100 degreeC, and stirred. Separately, a solution obtained by adding the ruthenium carbene complex represented by the above formula (11) (0.416 mg, 0.635 μmol) to toluene (0.55 g) was prepared. To the aforementioned monomer solution, the ruthenium carbene complex catalyst solution was added to start the polymerization reaction. After 1 hour of polymerization, a toluene (0.55 g) solution of ethyl vinyl ether (0.046 mg, 0.635 μmol) was added as a reaction stopper to obtain a ring-opened copolymer solution. The conversion rate of the monomer was measured and found to be 99% by mass. A ring-opened copolymer was obtained by precipitating a part of it in a large amount of methanol and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Abbe number | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| Abbe number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com