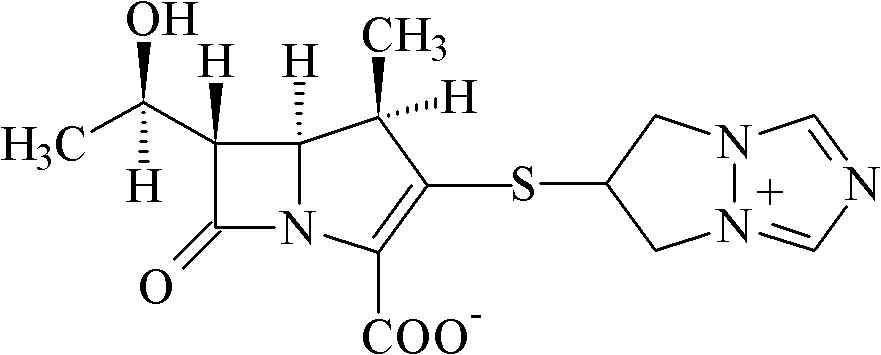
Biapenem crystalline solid and preparation method thereof
A technology of crystals and crystal forms, applied in the field of biapenem crystals and its preparation, can solve the problems of drug safety, effectiveness, stability of physical properties, etc., and achieve solid-liquid separation, low cost, and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0053] Experimental example 1 Experiment of influencing factors of biapenem crystals
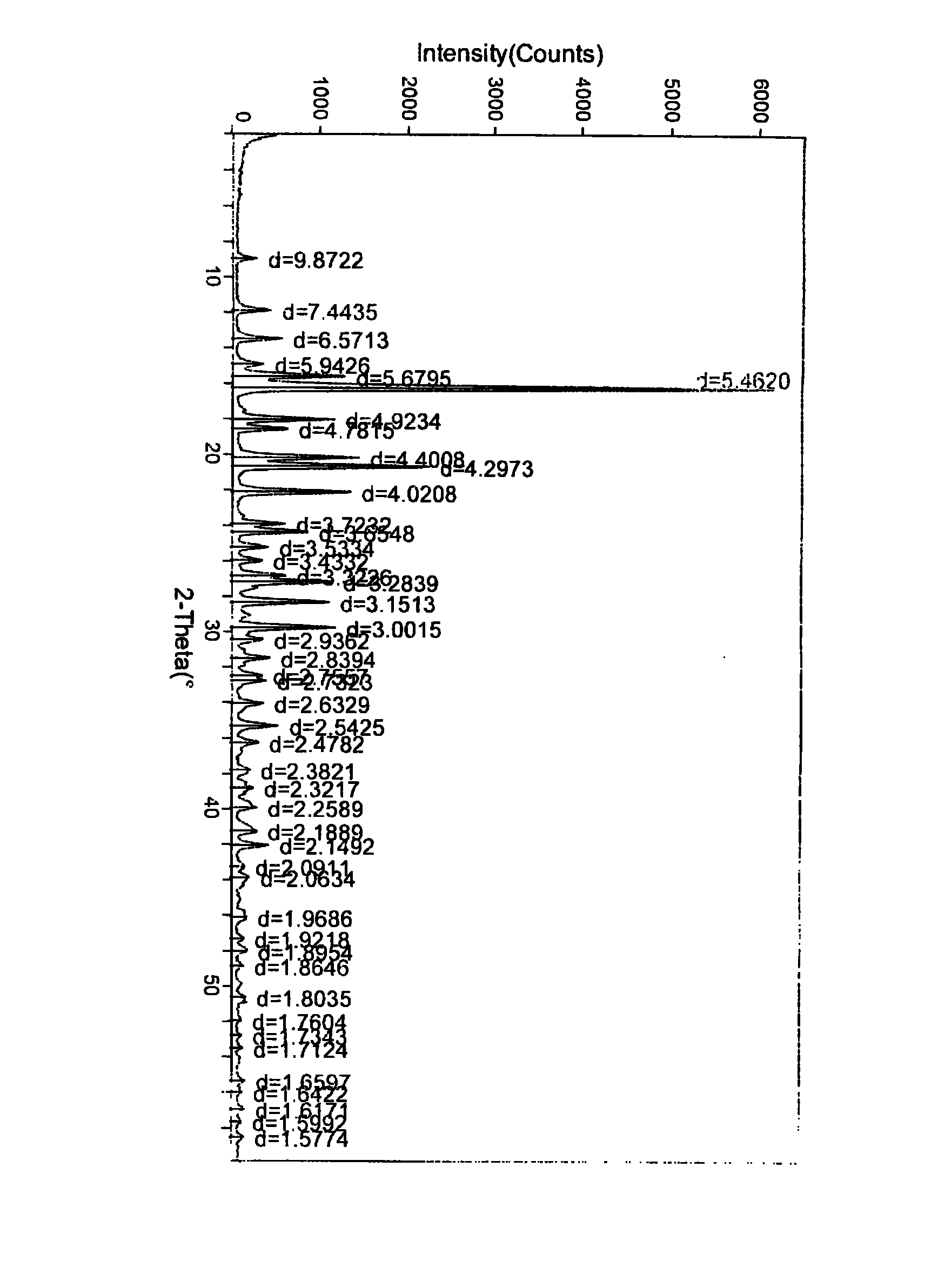
[0054] Put 10g of crude biapenem into 500ml of water for injection (control the temperature at 5°C to 15°C), stir and dissolve completely, add 2g of activated carbon, stir for 40 minutes to decolorize and filter, filter the solution, then add ethanol under stirring 3000ml, stirred and controlled temperature (5°C-15°C) for 3 hours to crystallize, dried to obtain 8.2g white crystals, decomposition temperature 230°C-232°C, HPLC purity 99.8%, powder X-ray was carried out on the obtained biapenem crystals Diffraction analysis, the results are attached figure 1 ,Table 1.
[0055] Table 1 Powder X-ray Diffraction Data of Biapenem Crystals
[0056] 2θ value
[0057] The obtained white crystals of biapenem were placed under conditions of high temperature, light, and high humidity (92.5%) for 10 days. Compared with 0 days, the total impurities under each condition were as follows, and the ...
experiment example 2
[0061] Experimental Example 2 Stability Investigation
[0062] The crystalline finished product prepared in Example 1 and the self-made amorphous finished product were placed under light (4500lx) for 10 days, high temperature 60°C for 10 days, and high humidity (92.5%) for 10 days, respectively. 1. Comparing the influence of the amorphous powder of apenem and the crystalline product of biapenem prepared in Example 1 under high humidity and strong light conditions. The specific experimental data are shown in Table 3 below.
[0063] Table 3 Experimental results of influencing factors of amorphous fraction and crystalline biapenem
[0064]
[0065]
[0066] It can be seen from Table 3 that the main quality indicators of the biapenem crystals obtained in the present invention: traits, related substances and contents are all stable to biapenem amorphous products under strong light, high temperature and high humidity conditions; Under the conditions, the amorphous product ha...
Embodiment 1
[0068] Example 1 Preparation method of biapenem crystals of the present invention
[0069] Put 10 g of crude biapenem into 500 ml of water for injection (control the temperature at 5°C to 15°C), stir and dissolve completely, add 2 g of activated carbon, stir for decolorization and filter for 40 minutes, filter the solution, and add acetic acid under stirring Ethyl ester was 3000ml, stirred and temperature-controlled (5°C-15°C) for 3 hours to crystallize, and dried to obtain 8.5g of white crystals with a decomposition temperature of 230°C-231°C and a HPLC purity of 99.8%. The obtained biapenem crystals were subjected to powder X - Ray Diffraction Analysis, the results are attached figure 1 ,Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com