Recombined Talpha 1-BP5 fusion peptide, gene, engineering bacteria and application
A 1-BP5 and fusion peptide technology, applied in the field of bioengineering, can solve the problems of high content of miscellaneous proteins, difficult purification, unsuitable for field promotion, etc., achieve broad application prospects, good immune adjuvant effect, and enhance the body's cellular immunity and the effect on humoral immunity levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1, Construction of Engineering Bacteria Expressing Recombinant Tα1-BP5 Fusion Peptide
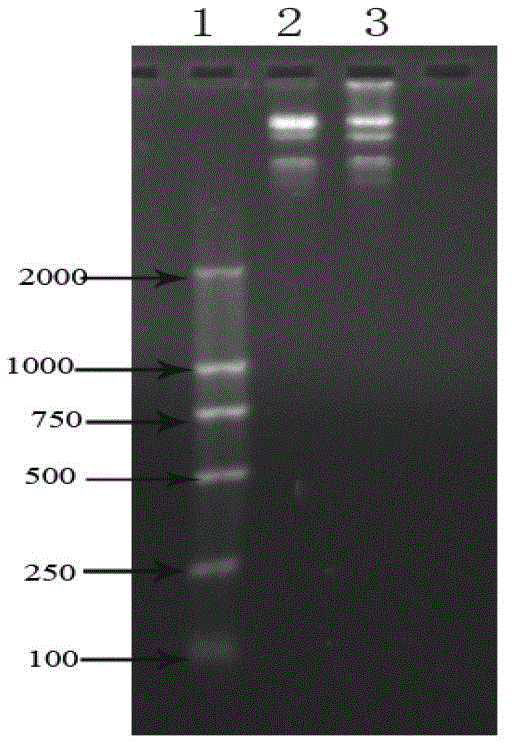
[0043] 1. SOE-PCR amplification of recombinant Tα1-BP5 fusion peptide gene
[0044] 1) Design primers
[0045] Design the recombinant Tα1-BP5 fusion peptide gene according to the biased codon of porcine Escherichia coli, as follows:
[0046] 5'-AGCGACGCTGCTGTTGACACTAGCAGCGAAATCACTACTAAAGACTTGAAAGAA AAAAAGAAGTTGTTGAAGAAGCTGAAAACGGCGGCGGCGGCAGCTGCAAAAATGTG TAT-3' (SEQ ID NO: 1)
[0047] And design primer F for this fusion peptide gene 1 , F 2 and F 3 , where in primer F 1 Add EcoRI restriction site, primer F 3 Add a stop codon and a SalI restriction site, and the primers were synthesized by Shanghai Invitrongen Company, as follows:
[0048] f 1 : 5'-CCG GAATTC AGCGACGCTGCTGTTGACACTAGCAGCGAAATCACTACTAAAG ACTTG-3' (SEQ ID NO: 2), wherein the underlined part is the EcoRI restriction site;
[0049] f 2 : 5'-GTTCGGGGTGCTGCCGCCGCCGCCGTTTTCAGCTTCTTCAACAACTTCTTTTTTTTCTTTCAA...
Embodiment 2
[0058] Embodiment 2, preparation of recombinant Tα1-BP5 fusion peptide
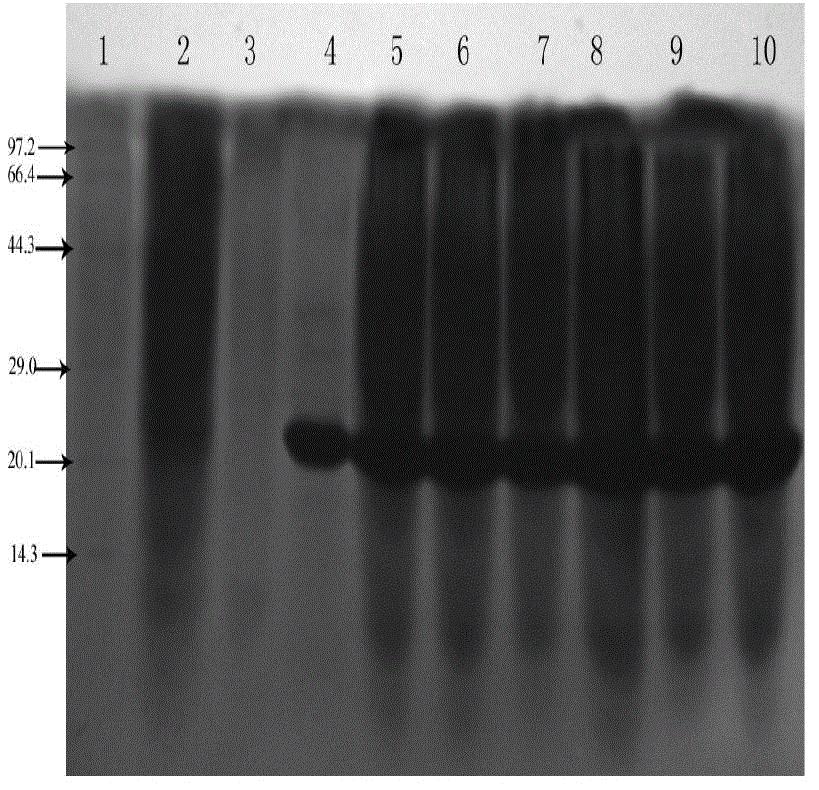
[0059] 1. Inoculate the engineered bacteria expressing the recombinant Tα1-BP5 fusion peptide into 3mL LB liquid medium (50μg / mL ampicillin), and cultivate overnight at 37°C with shaking; the next day, take out the bacterial liquid and add it to 3mL LB liquid culture base, so that the cell concentration reached OD 600 After ≈0.1, shake culture at 37°C, when the cell concentration OD 600 When ≈0.4-0.6, add IPTG to induce expression for 4 hours, the final concentration of IPTG is 1mM; collect 100 μL of bacterial liquid every 1 hour, and perform SDS-PAGE on the expression of recombinant Tα1-BP5 fusion peptide in E. coli at different induction times analysis, such as figure 2 shown. Centrifuge the bacterial solution at 4000rpm for 10min, collect the bacterial cells, resuspend the collected bacterial cells with 100μL PBS, add an equal volume of 2×SDS gel loading buffer (100mmol / L Tris Cl (pH6.8), 200mmol ...
experiment example 1
[0067] Experimental example 1, in vitro activity determination of recombinant Tα1-BP5 fusion peptide
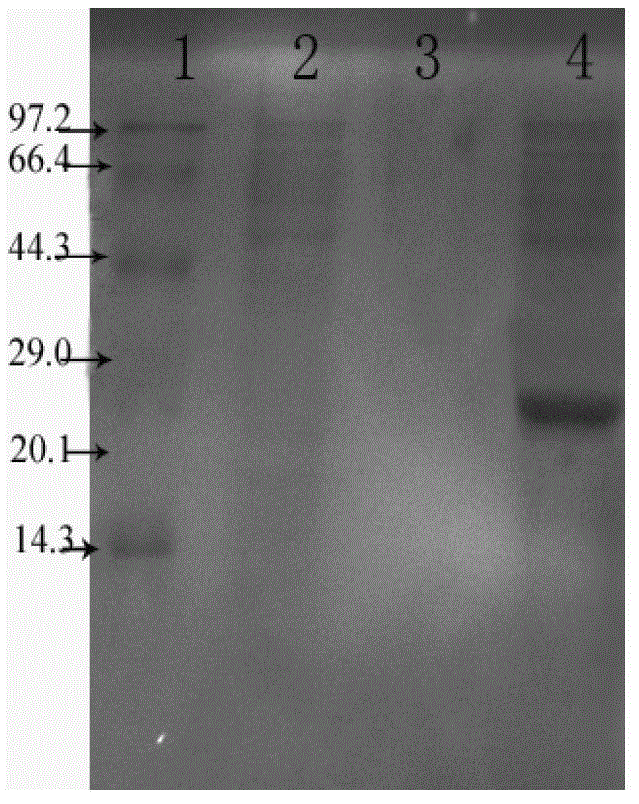
[0068] Mouse lymphocytes were isolated by conventional methods and placed in CO 2 Incubate in an incubator at 37°C for 6 h; add different concentrations of recombinant Tα1-BP5 fusion peptides (20 μmoL / L, 10 μmoL / L, 5 μmoL / L, 2.5 μmoL / L, 1.25 μmoL / L) into wells containing lymphocytes, each The well was 100 μL, and a control group (PBS, 10 μmoL / L thioredoxin (TRx), 10 μmoL / L standard Tα1, 10 μmoL / L BP5) was set up, and the in vitro activity of the recombinant Tα1-BP5 fusion peptide was determined by MTT method. The results are as follows: Figure 4 shown. From Figure 4 It can be seen that the recombinant Tα1-BP5 fusion peptide of the present invention can significantly promote the division and proliferation of lymphocytes. Compared with single thymosin α1 and bursa pentapeptide BP5, the recombinant Tα1-BP5 fusion peptide of the present invention stimulates The proliferative...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com