7,4'-disubstituted isoflavone derivative and preparation method and application thereof
A technology of isoflavones and derivatives, applied in the field of medicinal chemistry, can solve the problems of not well developed antitumor activity of isoflavones, and the improvement of antitumor activity is not obvious.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
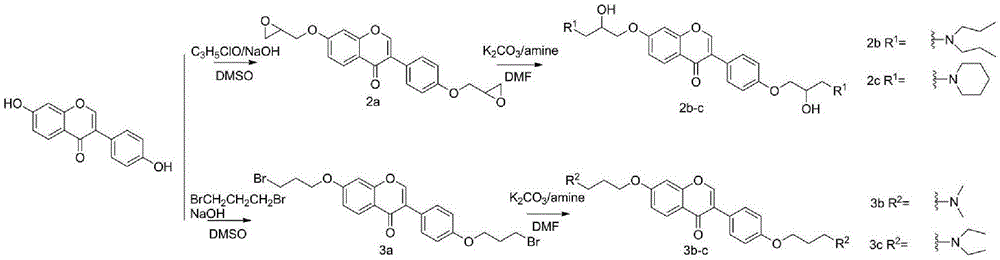
[0021] Example 1: Synthesis of 7,4'-two (2,3-epoxypropoxyl) isoflavones
[0022] Dissolve 7,4'-dihydroxyisoflavone (10g, 0.0394mol) in 150ml DMSO, add NaOH (3.2g, 0.08mol) and epichlorohydrin (16ml, 0.2mol), and react at 70°C overnight to saturate saline and CH 2 Cl 2 Extraction, and column chromatography to separate the crude product to obtain 7,4'-bis(2,3-epoxypropoxy)isoflavone. Pale yellow solid, yield 55%. 1 H NMR (400MHz, CDCl 3 )δ8.15(d,J=8.9Hz,1H),7.85(s,1H),7.42(t,J=5.8Hz,2H),6.95(dd,J=9.0,2.4Hz,1H),6.92( d,J=8.8Hz,2H),6.82(d,J=2.3Hz,1H),4.33–4.16(m,2H),3.94(td,J=11.1,5.8Hz,2H),3.37–3.28(m ,2H),2.87(dt,J=15.0,4.5Hz,2H),2.72(ddd,J=10.6,4.8,2.6Hz,2H). 13 C NMR (101MHz, CDCl 3 )δ175.78,162.70,158.49,157.79,152.21,130.19,127.97,124.82,124.75,118.79,114.73,114.68,101.06,69.31,68.81,50.15,49.79,458.76,44 21 h 18 o 6 :C68.85,H4.95;Found C68.81,H4.96.
Embodiment 2
[0023] Example 2: Synthesis of 7,4'-bis(2-hydroxyl-3-aminopropoxy)isoflavones
[0024] 7,4'-bis(2,3-epoxypropoxy)isoflavone (0.4g, 0.00109mol) was dissolved in 50mlCHCl 3 , add potassium carbonate (0.5g, 0.0036mol) and secondary amine (0.01mol), react at 70 ° C for 24 hours, with saturated saline and CH 2 Cl 2 Extraction and column chromatography to separate the crude product to obtain 7,4'-bis(2-hydroxy-3-aminopropoxy)isoflavone.
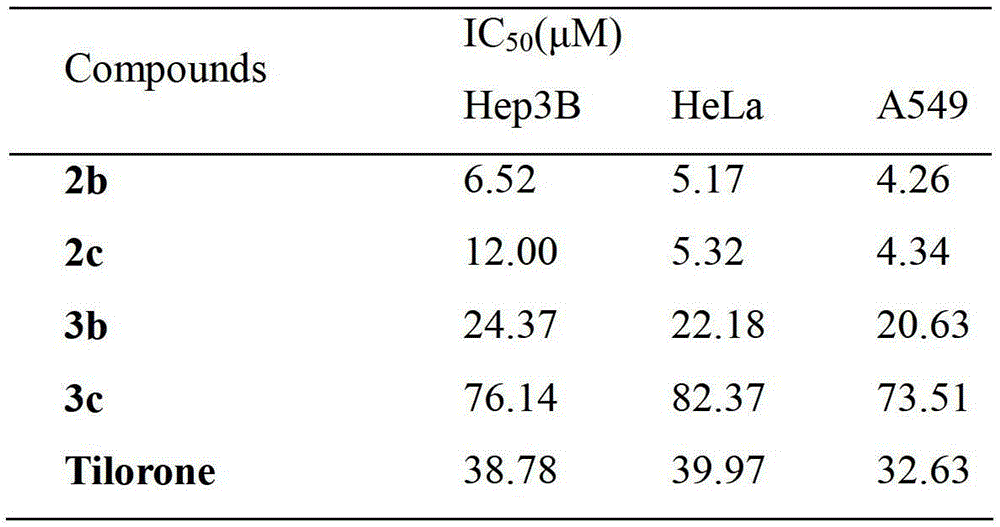
[0025] 7,4'-bis(2-hydroxy-3-di-n-aminopropoxy)isoflavone (2b). Pale yellow oily liquid, yield 65%. 1 H NMR (400MHz, CDCl 3 )δ:8.20(d,J=8.9Hz,1H),7.92(s,1H),7.49(d,J=8.7Hz,2H),7.07–6.96(m,3H),6.89(d,J=2.3 Hz,1H),4.10–3.97(m,6H),2.64–2.40(m,12H),1.59–1.41(m,8H),0.90(td,J=7.3,2.5Hz,12H). 13 C NMR (101MHz, CDCl 3 )δ:175.86,163.18,158.86,157.84,152.13,130.10,127.78,124.84,124.43,118.55,114.84,114.60,100.82,71.02,70.55,65.92,65.70,57.23,56.93,56.25,56.22,20.30,20.29,11.80 .Anal.Calcd.for C 33 h 48 N 2 o 6 :C69.69,H8.51N4.93;Found C69.65,H8.52...
Embodiment 3
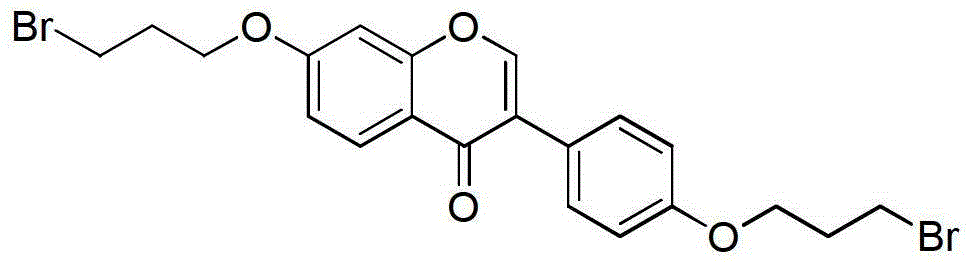
[0027] Example 3: Synthesis of 7,4'-bis(3-bromopropoxy)isoflavones
[0028] Dissolve 7,4'-dihydroxyisoflavone (5g, 0.0197mol) in 100ml DMSO, add NaOH (1.6g, 0.04mol) and 1,3-dibromopropane (10ml, 0.1mol), and react at 70°C overnight. The reaction system was poured into 1M NaOH aqueous solution, the precipitate was collected, washed and dried, and the crude product was separated by column chromatography to obtain 7,4'-bis(3-bromopropoxy)isoflavone. Pale yellow solid, yield 70%. 138-141°C. 1 H NMR (400MHz, CDCl 3 )δ:8.22(d,J=8.9Hz,1H),7.92(d,J=2.8Hz,1H),7.53–7.46(m,2H),7.02–6.95(m,3H),6.88(d,J =2.3Hz,1H),4.19(dt,J=29.9,5.8Hz,4H),3.63(dd,J=11.4,6.3Hz,4H),2.43–2.29(m,4H). 13 C NMR (101MHz, CDCl 3)δ:175.85,163.00,158.69,157.90,152.12,130.20,127.90,124.89,124.48,118.59,114.76,114.59,100.76,65.94,65.37,32.33,31.98,30.Calc.92,29 21 h 20 Br 2 o 4 :C50.83,H4.06;Found C50.78,H4.05.MS(ESI):m / z[M-Br] + calcd415.1, found414.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com