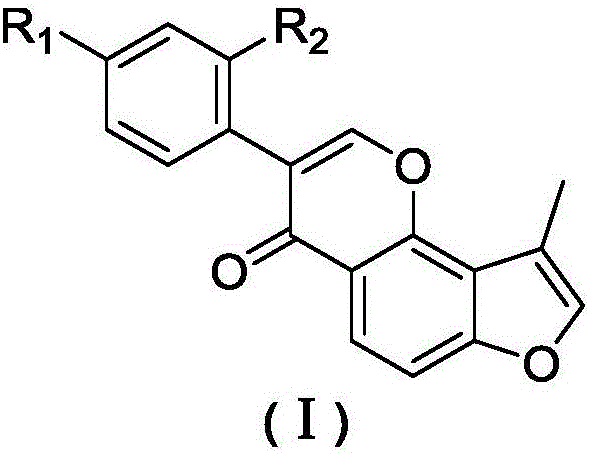
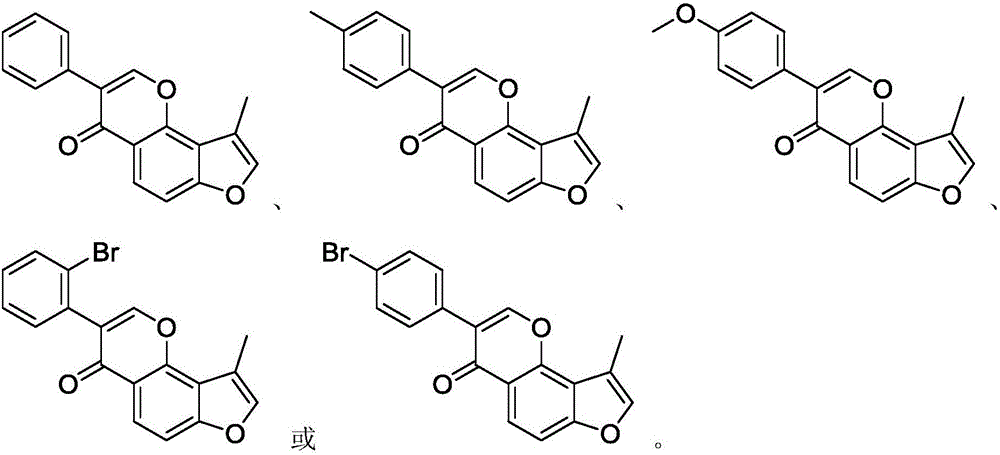
Substituted furan isoflavone derivative and preparation method thereof
A technology of furan isoflavones and derivatives, which is applied in the field of medicinal chemistry, can solve problems that have not been reported, and achieve the effect of simple steps and low loss rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
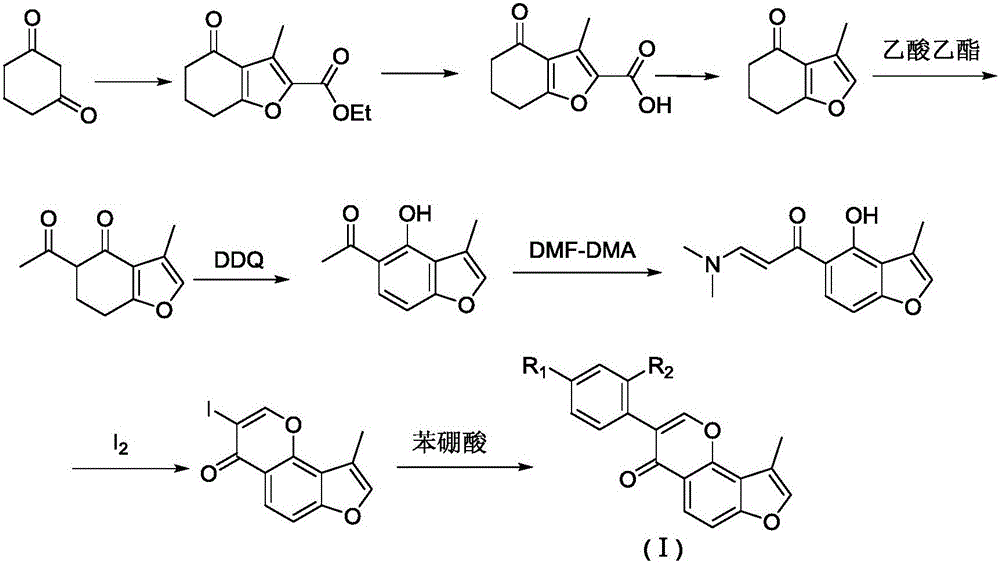
[0035] Disperse 1,3-cyclohexanedione and potassium hydroxide uniformly in water, stir at room temperature for 5 minutes, add methanol solution of ethyl chloroacetoacetate, then stir the system at room temperature for 5 days, and then use 4N Acidify with hydrochloric acid, filter the acidified reaction solution, and the obtained solid is the product: ethyl 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylate. In this step, the molar ratio of 1,3-cyclohexanedione to potassium hydroxide and ethyl chloroacetoacetate is 1:1:1, and the feeding amount of 1,3-cyclohexanedione corresponding to every milliliter of water is 0.1g , the feeding amount of ethyl chloroacetoacetate corresponding to every milliliter of methanol is 0.2g. The yield for this step was 65%. The structure of the resulting product is:
[0036]
[0037] Molecular formula: C 12 h 14 o 4
[0038] Chinese name: ethyl 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylate
[0039] English name: ethyl 3-m...
Embodiment 2
[0044]Dissolve ethyl 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylate and potassium hydroxide in a mixed solvent of methanol and water, and stir the system at room temperature after dissolution Reaction 5h. Then adjust the pH of the reaction solution to 1 with 6N hydrochloric acid, filter the reaction solution, and filter the obtained solid as the product: 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2- formic acid. The mixed solvent used in this step is methanol and water at a ratio of 2.5:1. The feeding amount of ethyl 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylate per milliliter of mixed solvent is 0.2 g. In this step, the molar ratio of ethyl 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylate to potassium hydroxide was 1:6. The yield for this step was 90%. The structure of the resulting product is:
[0045]
[0046] Molecular formula: C 10 h 10 o 4
[0047] Chinese name: 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylic acid
...
Embodiment 3
[0053] Disperse 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylic acid evenly in diethylene glycol, add copper powder and pyridine, then heat the system to 175°C, Keep stirring for 10h. Cool the system to room temperature, add ice water, and acidify with 4N hydrochloric acid, extract the acidified reaction solution with petroleum ether three times, wash the combined extract with water once, then dry the extract with anhydrous sodium sulfate, and spin dry. The obtained solid is the product: 3-methyl-6,7-dihydrobenzofuran-4-(5H)-one. In this step, the feeding amount of 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylic acid per milliliter of diethylene glycol is 0.1 g. In this step, the molar ratio of 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylic acid to copper powder and pyridine is 1:1:2. The yield for this step was 85%. The structure of the resulting product is:
[0054]
[0055] Molecular formula: C 9 h 10 o 2
[0056] Chinese name: 3-methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com