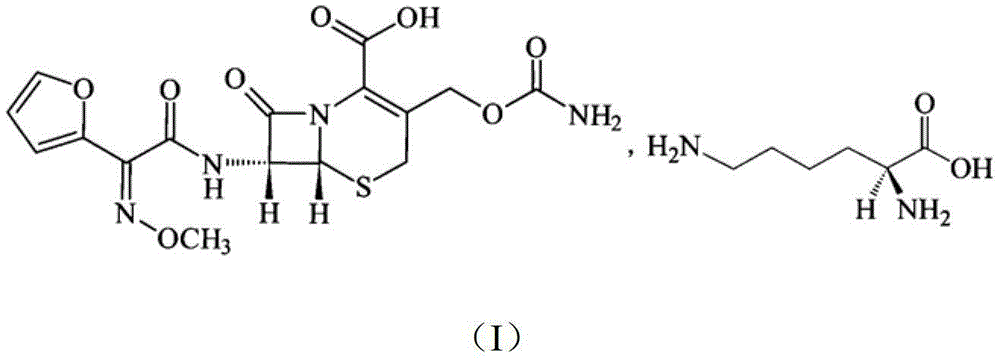
Cefuroxime lysine medicinal composition
A cefuroxime lysine and composition technology, applied in the field of cefuroxime lysine, can solve the problem of less than 99% purity, and achieve the effects of no solvent residue, improved stability, and improved curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Add cefuroxime lysine solid to 100ml of distilled water at 25°C to prepare a saturated solution of cefuroxime lysine solid;
[0043] (2) In a sound field with a frequency of 25KHz and an output power of 30W, add a mixed solution of absolute ethanol and ethyl acetate at 0°C and stir while adding at a stirring speed of 600 rpm; absolute ethanol and ethyl acetate The volume ratio of the ester is 1:0.2, the volume of absolute ethanol and ethyl acetate is 200ml, and the adding speed is v=3.33ml / min;
[0044] (3) Remove the sound field after adding the mixed solvent, and let the crystal grow at 5°C for 5 hours;
[0045] (4) Filtrate, wash the filter cake with ethanol, and dry in vacuum for 3 hours to obtain cefuroxime lysine crystals.
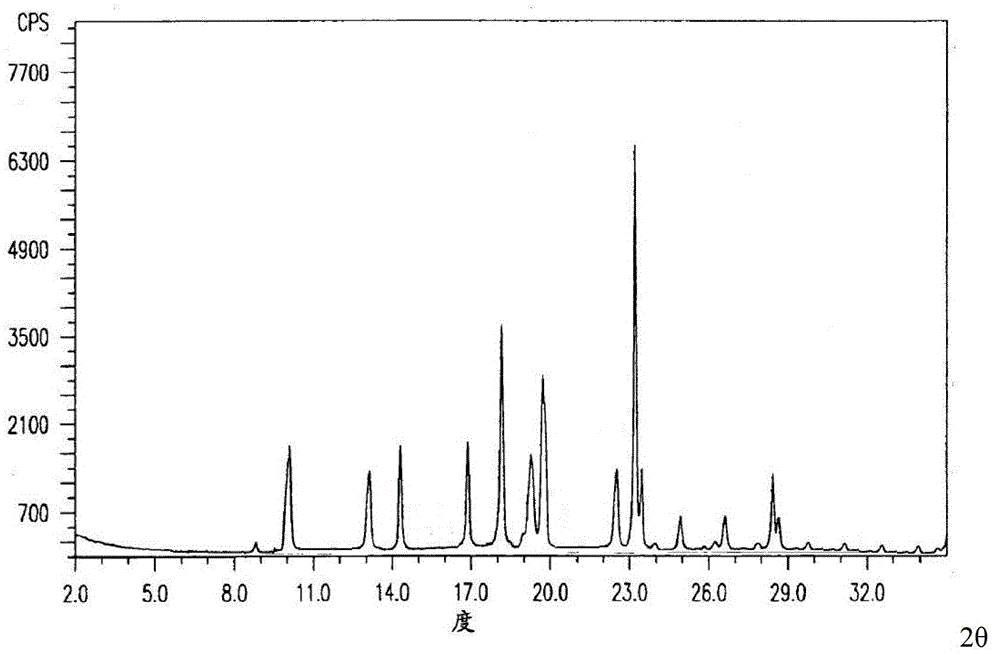
[0046] The prepared cefuroxime lysine crystal obtained by Cu-Kα ray measurement X-ray powder diffraction pattern is as follows figure 1 Shown; The main particle size is 350-600 μm and the distribution width is 250-800 μm through scanning...
Embodiment 2
[0048] (1) Add cefuroxime lysine solid to 600ml of distilled water at 30°C to prepare a saturated solution of cefuroxime lysine solid;
[0049] (2) In a sound field with a frequency of 30KHz and an output power of 40W, add a mixed solution of absolute ethanol and ethyl acetate at 1°C, stir while adding, and the stirring speed is 150 rpm; absolute ethanol and ethyl acetate The volume ratio of the ester is 1:0.1, the volume of absolute ethanol and ethyl acetate is 2400ml, and the adding speed is 5ml / min;
[0050] (3) Remove the sound field after adding the mixed solvent, and let the crystal grow at 15°C for 5 hours;
[0051] (4) Filtrate, wash the filter cake with ethanol, and dry in vacuum for 6 hours to obtain cefuroxime lysine crystals.
[0052] The prepared cefuroxime lysine crystal obtained by Cu-Kα ray measurement X-ray powder diffraction pattern is as follows figure 1 Shown; The main particle size is 350-600 μm and the distribution width is 250-800 μm through scanning e...
Embodiment 3
[0054] (1) Add cefuroxime lysine solid to 300ml of distilled water at 45°C to prepare a saturated solution of cefuroxime lysine solid;
[0055] (2) In a sound field with a frequency of 30KHz and an output power of 30W, add a mixed solution of absolute ethanol and ethyl acetate at 1°C, stir while adding, and the stirring speed is 120 rpm; absolute ethanol and ethyl acetate The volume ratio of the ester is 1:0.2, the volume of absolute ethanol and ethyl acetate is 900ml, and the adding speed is 2ml / min;
[0056] (5) Remove the sound field after adding the mixed solvent, and let the crystal grow at 8°C for 5 hours;
[0057] (6) Filtrate, wash the filter cake with ethanol, and dry in vacuum for 6 hours to obtain cefuroxime lysine crystals.
[0058] The prepared cefuroxime lysine crystal obtained by Cu-Kα ray measurement X-ray powder diffraction pattern is as follows figure 1 Shown; The main particle size is 350-600 μm and the distribution width is 250-800 μm through scanning ele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Primary particle size | aaaaa | aaaaa |
| Distribution width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com