Preparation method and application of prodrug of endopeptidase activated doxorubicin
A technology of doxorubicin and endopeptidase, which is applied in the fields of biology and medicine, can solve the problems of long time consumption, low product yield, low stability and purity, low reaction rate, etc., and achieves high yield and simple and easy synthesis method. performance, improve curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
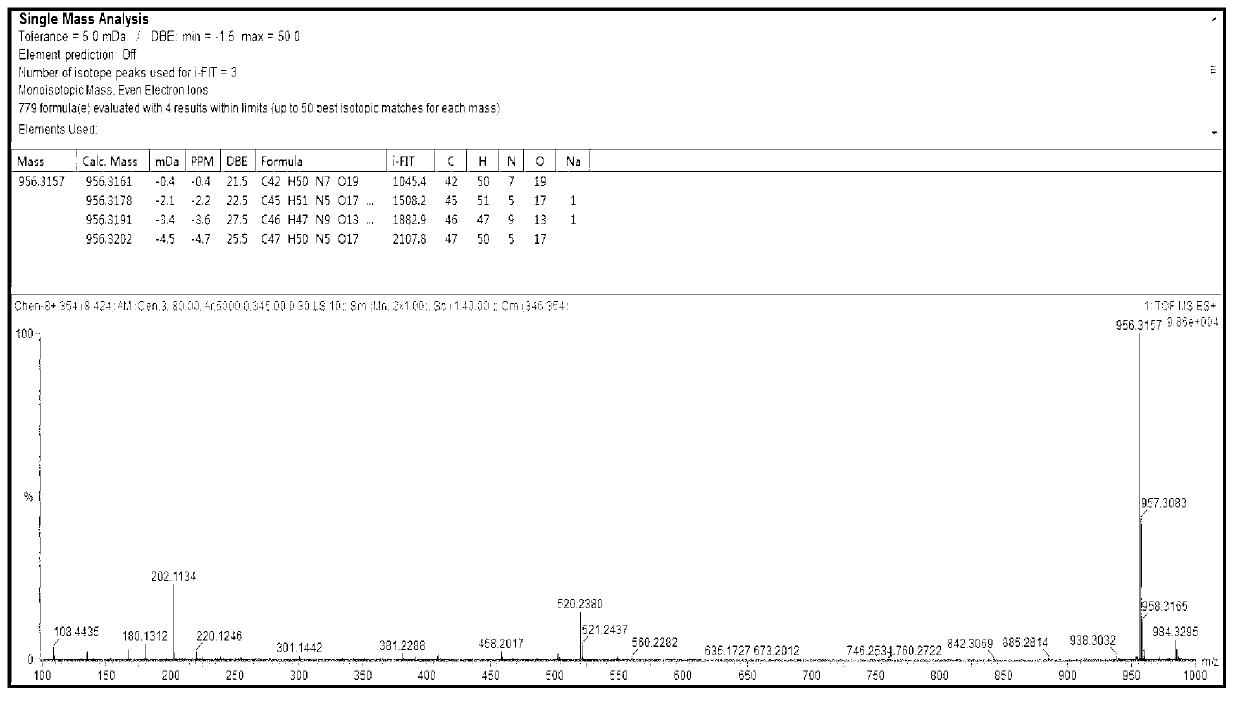
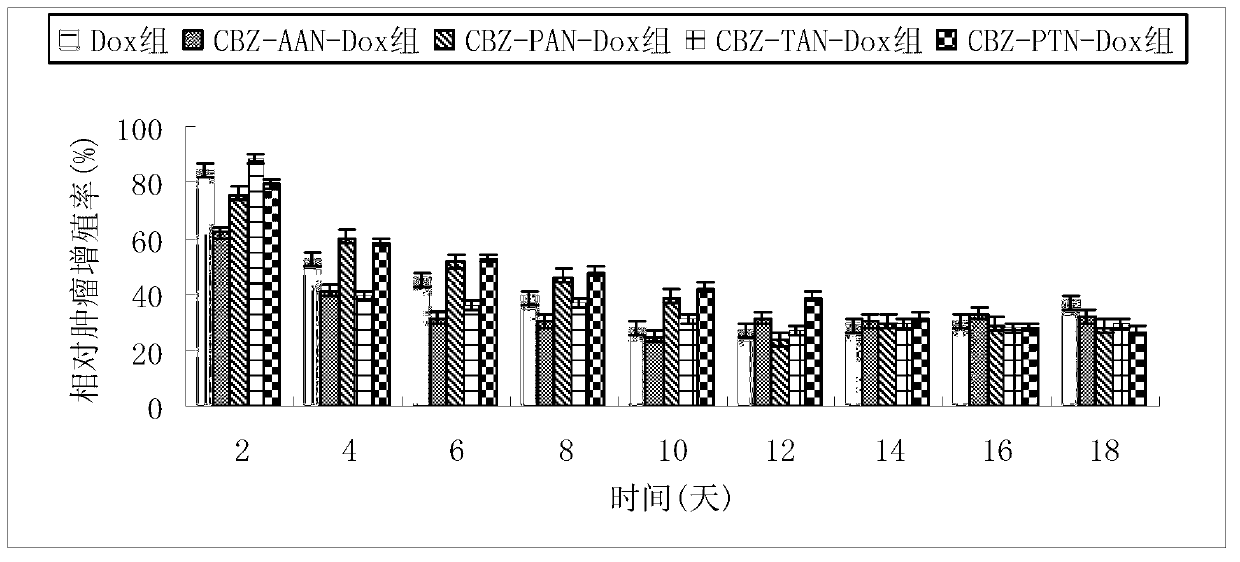
[0035] Example 1: Design and synthesis of N-benzyloxycarbonyl-alanyl-alanyl-asparagine modified doxorubicin prodrug (CBZ-AAN-Dox)
[0036] (1) Activation of N-benzyloxycarbonyl-alanyl-alanyl-asparagine (CBZ-AAN-OH):
[0037] Weigh 39.0mg (95μmol) CBZ-AAN-OH (Guangzhou Jietewei Biotechnology Co., Ltd.) into a 25mL round bottom flask, add 12.9mg (95μmol) HOBt and 54.0mg (95μmol) HBTU, add 1.0mL DMF, Stir in an ice bath for 30 minutes, and when the temperature of the system drops to -15 to 5°C, add 9.5 μL (95 μmol) of NMM and react for 15 minutes.
[0038] (2) Preparation of CBZ-AAN-Dox:
[0039] Add 50 mg (86 μmol) of doxorubicin hydrochloride to the system in step (1), and add 1.0 mL of DMF, and react at room temperature until the fluorescence intensity of the product point is no longer increased by thin-layer chromatography (TLC), stop the reaction, and extract with ether The complex of tripeptide covalently coupled with doxorubicin was obtained by rotary evaporation.
[00...
Embodiment 2
[0042] Example 2: Design and synthesis of N-benzyloxycarbonyl-prolyl-alanyl-asparagine modified doxorubicin prodrug (CBZ-PAN-Dox)
[0043] (1) Activation of N-benzyloxycarbonyl-prolyl-alanyl-asparagine (CBZ-PAN-OH):
[0044] Weigh 41.5mg (95μmol) of CBZ-PAN-OH (Guangzhou Jietewei Biotechnology Co., Ltd.) into a 25mL round bottom flask, add 12.9mg (95μmol) of HOBt and 54.0mg (95μmol) of HBTU, add 1.0mL of DMF, Stir in an ice bath, and when the temperature of the system drops to -15-5°C, add 20 μL (200 μmol) of NMM and react for 10 minutes.
[0045] (2) Preparation of CBZ-PAN-Dox:
[0046] Add 55mg (95μmol) doxorubicin hydrochloride to the system in step (1), and add 1.0mL DMF, react at room temperature, until the fluorescence intensity of the product point is no longer increased by thin layer chromatography (TLC), stop the reaction, diethyl ether The extract was subjected to rotary evaporation to prepare a tripeptide covalently coupled doxorubicin complex.
[0047] (3) Purif...
Embodiment 3
[0049] Example 3: Design and synthesis of N-benzyloxycarbonyl-threonyl-alanyl-asparagine modified doxorubicin prodrug (CBZ-TAN-Dox)
[0050] (1) Activation of N-benzyloxycarbonyl-threonyl-alanyl-asparagine (CBZ-TAN-OH):
[0051] Weigh 41.8mg (95μmol) of CBZ-TAN-OH (Guangzhou Jietewei Biotechnology Co., Ltd.) into a 25mL round bottom flask, add 12.9mg (95μmol) of HOBt and 54.0mg (95μmol) of HBTU, add 1.0mL of DMF, Stir in an ice bath, and when the temperature of the system drops to -15-5°C, add 50 μL (500 μmol) of NMM and react for 8 minutes.
[0052] (2) Preparation of CBZ-TAN-Dox:
[0053] Add 37 mg (63 μmol) of doxorubicin hydrochloride to the system in step (1), and add 1.0 mL of DMF, react at room temperature, until the fluorescence intensity of the product point is no longer increased by thin-layer chromatography (TLC), stop the reaction, and extract with ether The complex of tripeptide covalently coupled with doxorubicin was obtained by rotary evaporation.
[0054] (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com