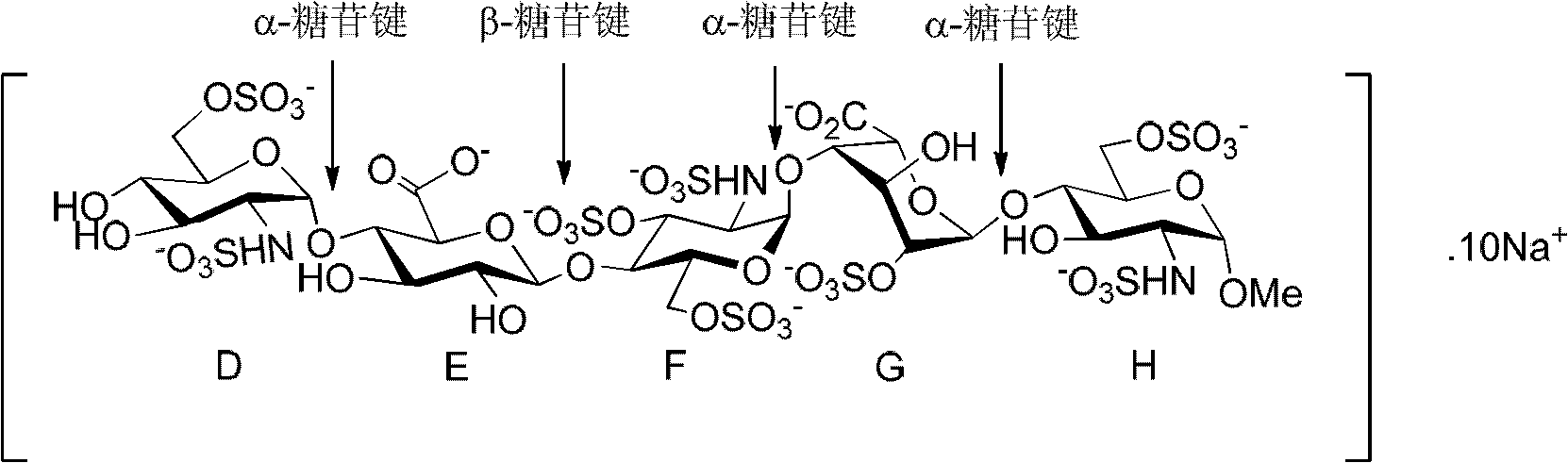
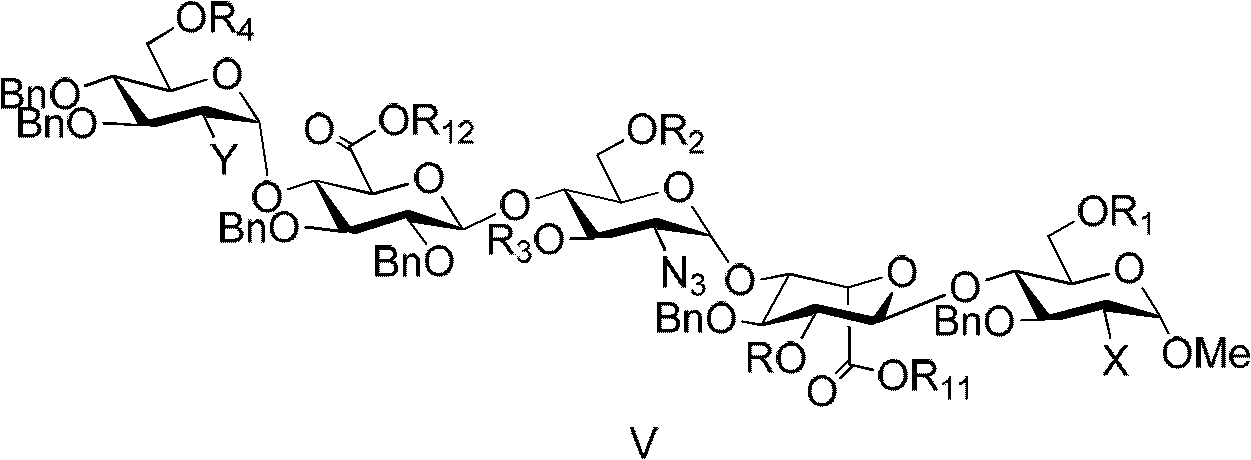
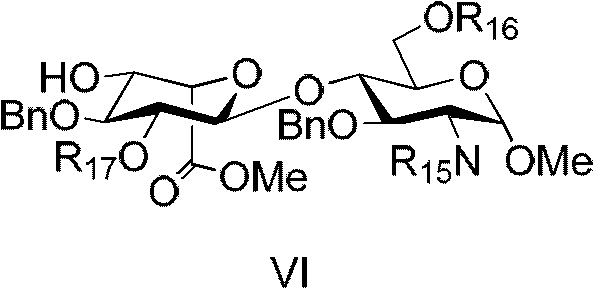
Compound for preparing fondaparinux sodium, preparation method thereof and preparation method of fondaparinux sodium
A compound and stereochemical technology, applied in the production of sugar derivatives, organic chemistry, bulk chemicals, etc., can solve the problems of low yield and complicated steps of fondaparinux
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086]
[0087] Heat 8.5g (2.95mmol) of phosphotungstic acid at 80°C for 0.5h under vacuum of 4 mm Hg, cool down naturally to 40°C under vacuum, and pass through N 2, add 2.5g (5.8mmol) GBn (can use Carbohydrate Research 2003, 338, 681-686 shown synthetic compound 6 similar method to prepare) and 3g (7.3mmol) HBz (can use Angew.Chem.Int.Ed .Engl.1993.32.1671-1690 page 1674~1675 shown in the synthetic compound 10a similar method to be prepared), anhydrous and anaerobic operation, N 2 50ml of toluene was added under low temperature, the temperature was lowered to -5°C, 0.2ml (1.1mmol) of TMSOTf was added, and kept at 0°C for 3h, then kept at + ].
Embodiment 2
[0089] Heat 3.3g of phosphotungstic acid at 80°C and 4mm Hg vacuum for 0.5h, then naturally cool down to 40°C under vacuum, and pass through N 2 , add 2.5g (5.8mmol) GBn and 3g (7.3mmol) HBz, anhydrous and anaerobic operation, N 2 Add 50ml of toluene at low temperature, cool down to -5°C, add 0.31ml of TMSOTf, keep at 0°C for 3h, then keep the temperature + ].
Embodiment 3
[0091] N 2 2.5gGBn and 3gHBz were dissolved in 50ml of dichloromethane, cooled to -5°C, TMSOTf 0.7ml (3.8mmol) was added, kept at 0°C for 3h, then kept at + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com