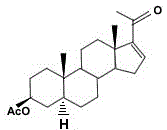
A kind of production method of monoenolone acetate synthesized by sisal saponin
A technology of monoenolone acetate and sisal saponin, which is applied in the chemical industry, can solve the problems of difficult centrifugation, incomplete reaction, low conversion rate and yield, etc., so as to improve product purity, reduce production cost, and provide production efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Throw 100g of sisal saponin, add 80g of acetic acid and 140g of acetic anhydride, and heat to carry out the ring-opening cracking reaction. The temperature is 218°C, the pressure is 0.65MPa and the ring opening is completed; the material is added to the oxidative hydrolysis reaction bottle containing 250g of acetic acid, and the temperature of the material is 12°C through -10~-5°C brine, and the mass concentration is 38 % of chromic anhydride oxidizing agent is added in the oxidation hydrolysis reaction bottle to carry out the oxidation reaction. The temperature rises to 98°C and the reaction time is 45 minutes. First, the acetic acid is recovered by atmospheric distillation and then the acetic acid is recovered under reduced pressure to 350g. After the reaction is completed, the solution after the reaction is ready for use;
[0032] 2) Add the solution obtained in step 1) into an extraction bottle, add 800 mL of cyclohexane into the extraction bottle, stir for 25 min...
Embodiment 2
[0036] 1) Throw 10kg of sisal saponin, add 8kg of acetic acid and 15kg of acetic anhydride, heat to carry out the ring-opening cracking reaction, when the temperature rises to 208°C and the internal pressure reaches 0.48MPa, turn off the power and time the ring-opening for 65 minutes, and finally react The inner temperature of the kettle is 225°C, and the pressure is 0.65MPa, and the ring opening is completed; the material is pressed into the oxidation hydrolysis tank filled with 25L acetic acid, cooled to 12°C with chilled water, and the chromic anhydride oxidant with a mass concentration of 38.5% is quickly added to the oxidation tank , carry out the oxidation reaction, the temperature rises to 98°C and the reaction is timed for 45 minutes. First, the acetic acid is recovered by distillation under normal pressure, and then the acetic acid is recovered under reduced pressure to 35L. After the reaction is completed, the solution after the reaction is ready for use;
[0037] 2) ...
Embodiment 3
[0041] 1) Throw 160kg of sisal saponin, add 192kg of acetic acid, 256kg of acetic anhydride, and 60kg of mother liquor to carry out the ring-opening cracking reaction. When the temperature rises to 210°C and the internal pressure reaches 0.49MPa, turn off the power and count the ring-opening for 65 minutes. Finally, the inner temperature is 225°C and the pressure is 0.65MPa, and the ring opening is completed; the material is pressed into the oxidation hydrolysis tank equipped with 400kg of acetic acid, cooled to 12°C with chilled water, and the chromic anhydride oxidant with a mass concentration of 40% is quickly added to the oxidation tank , carry out the oxidation reaction, the temperature rises to 98°C and the reaction is timed for 40 minutes, the acetic acid is first recovered by atmospheric distillation and then the acetic acid is recovered under reduced pressure to 550L, the reaction is completed, and the solution after the reaction is ready for use;
[0042] 2) Press the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com