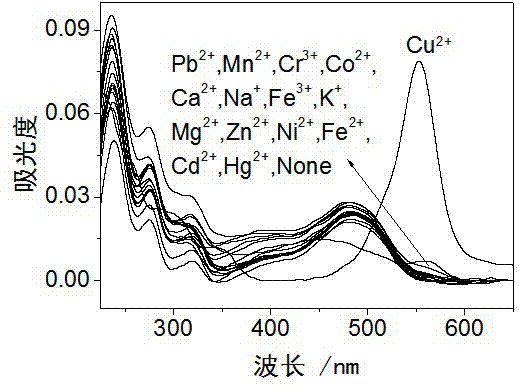
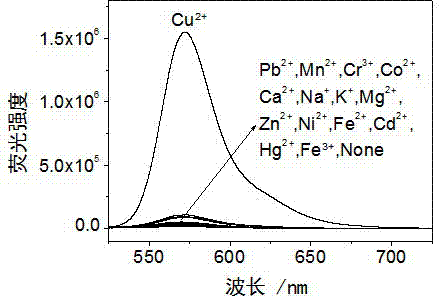
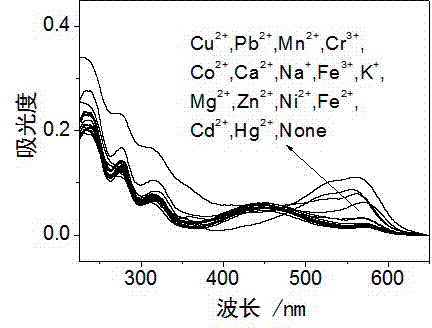
Rhodamine derivative and preparation method and application thereof
The technology of a derivative, rhodamine hydrazide, is applied in the field of rhodamine derivatives, achieving the effects of mild reaction conditions and short structural synthesis routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of intermediate rhodamine hydrazide (RBH)
[0036] The intermediate rhodamine hydrazide (RBH) was synthesized according to literature.
[0037] Dujols V, Ford F, Czarnik AW. A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. Am Chem Soc 1997;119:7386-7.
[0038] Yang X-F, Guo X-Q, Zhao Y-B. Development of a novel rhodamine-type fluorescent probe to determine peroxynitrite. Talanta 2002;57:883-90.
[0039] Wu C-M, Chen Y-H, Dayananda K, Shiue T-W, Hung C-H, Liaw W-F, Chen P-Y,Wang Y-M. Sensitivity evaluation of rhodamine B hydrazide towards nitric oxide and its application for macrophage cells imaging. Anal Chim Acta 2011;708:141- 8.
[0040] The specific operation is: in a 100 mL three-neck flask, dissolve 2 g rhodamine B (RB) in 25 mL ethanol, stir for 10 min, slowly add 6 mL of hydrazine hydrate with a dropping funnel, stir for another 10 min, and then heat to The reaction was refluxed for 6 h, and the reaction wa...
Embodiment 2
[0041] Example 2: Preparation of spectral probe RNBD
[0042] in dark and N 2 Under protective conditions, with acetonitrile as solvent, with anhydrous K 2 CO 3 is the acid-binding agent, the molar ratio n (RBH ) : n (acid-binding agent) : n (NBD-Cl) = 1:1:1. Dissolve RBH in acetonitrile in a 100 mL three-neck flask, stir for 10 min, then add anhydrous K 2 CO 3and NBD-Cl were dispersed in acetonitrile and slowly added to the RBH solution, stirred and reacted at 25 °C for 36 h, and the reaction solution was rotatably evaporated to remove acetonitrile, and the residue was first dispersed in deionized water, and then extracted with dichloromethane. The organic phase was taken, dried with anhydrous sodium sulfate, filtered to remove sodium sulfate, and the filtrate was rotary evaporated to remove the solvent to obtain a crude product. The crude product was subjected to silica gel column chromatography (the eluent was ethyl acetate / petroleum ether, volume ratio 1:5), and wash...
Embodiment 3
[0044] Example 3: Preparation of spectral probe RNBD
[0045] in dark and N 2 Under protective conditions, with acetonitrile as solvent, with anhydrous K 2 CO 3 is the acid-binding agent, the molar ratio n (RBH ) : n (acid-binding agent) : n (NBD-Cl) = 1.0:1.0:1.5. Dissolve RBH in acetonitrile in a 100 mL three-neck flask, stir for 10 min, then add anhydrous K 2 CO 3 and NBD-Cl were dispersed in acetonitrile and slowly added to the RBH solution, stirred and reacted at 25 °C for 36 h, and the reaction solution was rotatably evaporated to remove acetonitrile, and the residue was first dispersed in deionized water, and then extracted with dichloromethane. The organic phase was taken, dried with anhydrous sodium sulfate, filtered to remove sodium sulfate, and the filtrate was rotary evaporated to remove the solvent to obtain a crude product. The crude product was subjected to silica gel column chromatography (the eluent was ethyl acetate / petroleum ether, volume ratio 1:5), a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Slit width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com