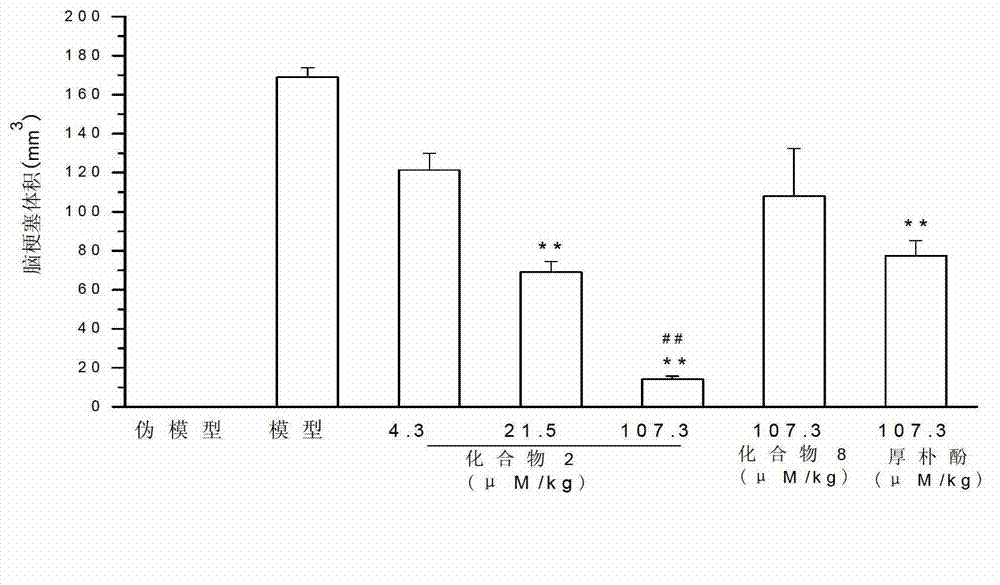
Magnolol derivative, honokiol derivative and preparation method and application thereof
A technology of phenol derivatives and magnolol, which is applied in the fields of magnolol derivatives and honokiol derivatives and their pharmaceutical applications, can solve the problems of poor water solubility, low bioavailability, and restrictions on wide application, and achieve good antibacterial Thrombosis, reducing the effect of cerebral ischemia or cerebral ischemia-reperfusion injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 [(S)-2,6-N-Boc-diamino-1-hexanoic acid]-[5,5'-dipropenyl-2'-hydroxy-2-biphenol] ester (compound 1) preparation of
[0052] Boc-Lys(Boc)-OH (3.097g, 8.9mmol) was dissolved in 80ml of dichloromethane, and DCC was added and stirred for 10min. Magnolol (1.9737 g, 7.3 mmol) was added to the solution and stirred at room temperature for 15 hours. The precipitate was filtered, the filtrate was concentrated, and residual column chromatography (PE:EA=10:1) obtained 2.3 g of compound 1.
[0053] Compound 1: C 34 h 46 N 2 o 7 =594.33, MS: 595.2[M+H] + . 1 H-NMR (300MHz, DMSO 3 )δ7.21-7.16 (2H, t), 7.11 (1H, s), 6.99-6.91 (2H, t), 6.81-6.76 (3H, t), 6.0-5.83 (2H, m), 5.09-4.98 ( 4H, m), 3.87-3.82 (1H, t), 3.39-3.23 (4H, m), 2.79 (2H, 1s), 1.37 (18H, s), 1.28-1.04 (8H, m).
Embodiment 2
[0054] Example 2 [(S)-2,6-diamino-1-hexanoic acid]-[5,5'-dipropenyl-2'-hydroxy-2-biphenol] ester hydrochloride (compound 2) preparation of
[0055] Add compound 1 (2.3g, 3.9mmol) and a saturated solution of hydrogen chloride in dioxane (50ml) into a 100ml single-necked bottle, stir at room temperature for 3 hours, remove the solvent under reduced pressure, and the crude product is subjected to column chromatography (C 18 , eluent: water / methanol=30%-50%) to obtain 1.7g of compound 2.
[0056] Compound 2: C 24 h 31 Cl 2 N 2 o 2 =449.18, MS: 395.2[M+H-2HCl] + . 1 H--NMR (300MHz, DMSO) δ9.44 (1H, s), 8.64 (3H, s), 8.00 (3H, s), 7.85-6.83 (6H, m), 6.08-5.86 (2H, m), 5.16-5.01 (4H, m), 4.01 (1H, m), 3.42-3.26 (4H, m), 2.62 (2H, m), 156-138 (4H, m), 117-099 (4H, m)
Embodiment 35
[0057] Example 35,5'-Dipropenyl-3-nitro-1,1'-biphenyl-2,2'-diphenol and 5,5'-dipropenyl-3,3'-dinitro- Preparation of 2,2'-biphenol (compounds 3a and 3b)
[0058] Add a solution of magnolol (0.5g, 1.88mmol) in acetic anhydride (500ml) into a 100ml single-necked bottle, and slowly add cold fuming nitric acid (5.9ml) and glacial acetic acid (6ml) dropwise at 0°C under constant stirring. anhydride (5ml) solution. After the dropwise addition, the reaction solution was poured into ice water, allowed to rise to room temperature, extracted with diethyl ether (500ml×3), dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure. The obtained crude product was separated by column chromatography (silica gel: 200-300 mesh, eluent: petroleum ether-petroleum ether / ethyl acetate 100:1) to obtain 150 mg of compound 3a and 250 mg of compound 3b.
[0059] Compound 3a: C 18 h 17 NO 4 =311.12, MS: 329[M+NH 4 ] + , 1 H-NMR (300MHz, CDCl 3 )δ8.01 (1H,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com