Application of diacylglycerol kinase alpha gene-chitosan nanoparticle in preparing medicine for treating allergic asthma
A chitosan nanoparticle, allergic asthma technology, applied in gene therapy, allergic diseases, drug combination, etc., can solve the problem of weakening T cell activation signal CD69 expression, T cell anergy, weakening Ras activation signal and inhibiting ERK phosphorylation issues such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
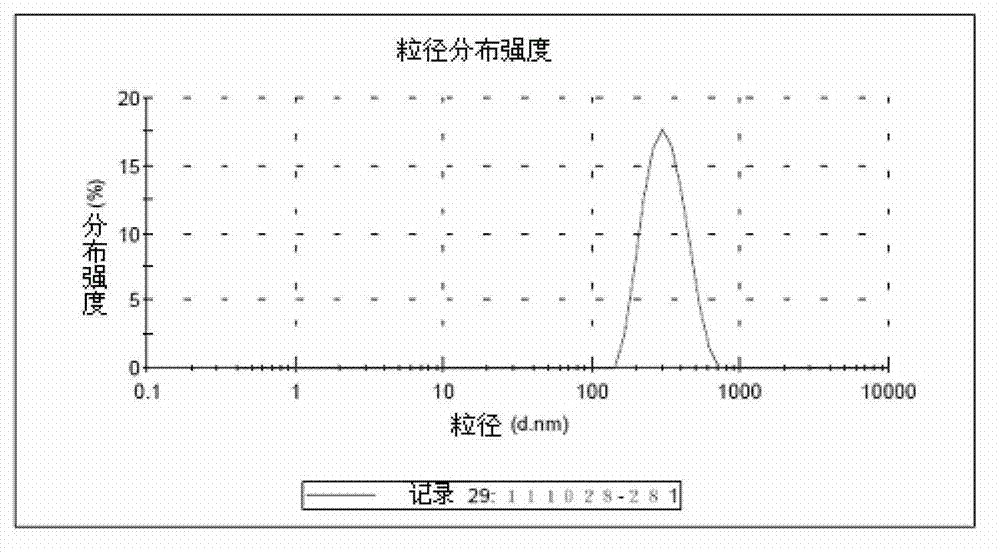
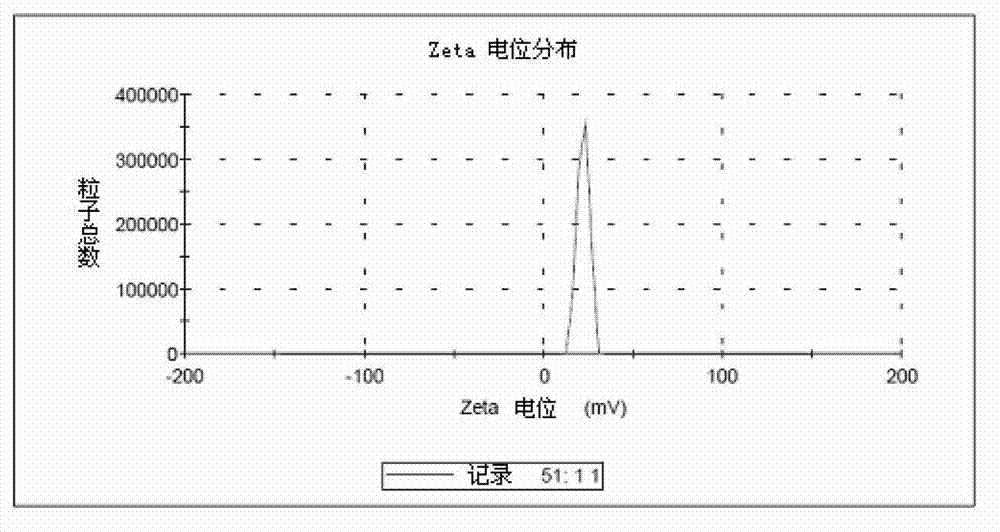
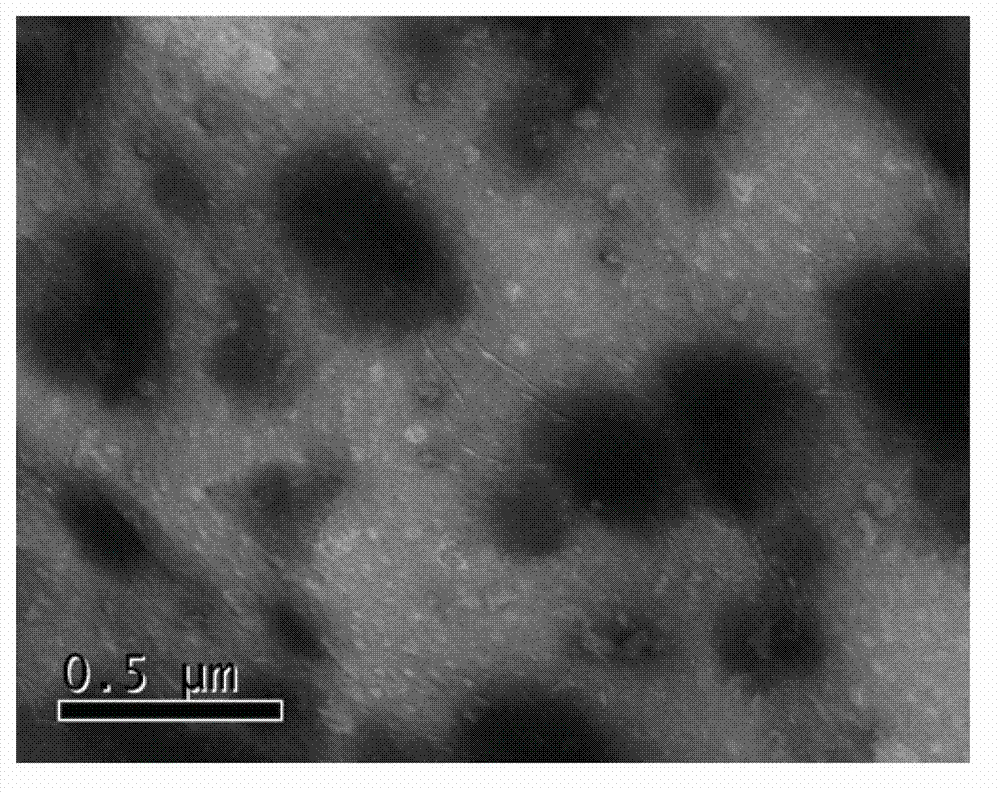
[0041] 1. Preparation of DGKα (Diglyceride Kinase α) Gene-Chitosan Nanoparticles:
[0042] Step 1. Fully synthesize the fusion gene of mouse IL-2 signal peptide sequence and mouse DGKα gene and clone into pEGFP-N3 vector to obtain DGKα plasmid. Sequencing confirmed that the mouse IL-2 signal peptide and the mouse DGKα gene sequence are completely consistent with the IL-2 (NM_008366.3) and DGKα gene (NM_016811.2) of GenBank;
[0043] Step 2. Dissolve chitosan in a solution with an acetic acid concentration of 1% to prepare a chitosan acetic acid solution with a concentration of 1.5 mg / ml, adjust its pH to 5.5 with sodium hydroxide, and then use a pore size of 0.22 μm sterile membrane filtration sterilization, in which the molecular weight of chitosan is 100,000 Daltons, and the degree of deacetylation is 95%;
[0044] Step 3. Dissolve the DGKα plasmid in 10mM 0.22μm sterile membrane-filtered anhydrous sodium sulfate solution to prepare a DGKα solution with a concentration of 360μg / ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com