Silver-Catalyzed Synthesis of Biheterocyclic Molecules and Fluorescent Biheterocyclic Molecules
A biheterocyclic and molecular technology, applied in the field of new structure biheterocyclic fluorescent molecules, can solve the problem of high synthesis cost and achieve the effect of high cross-coupling selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
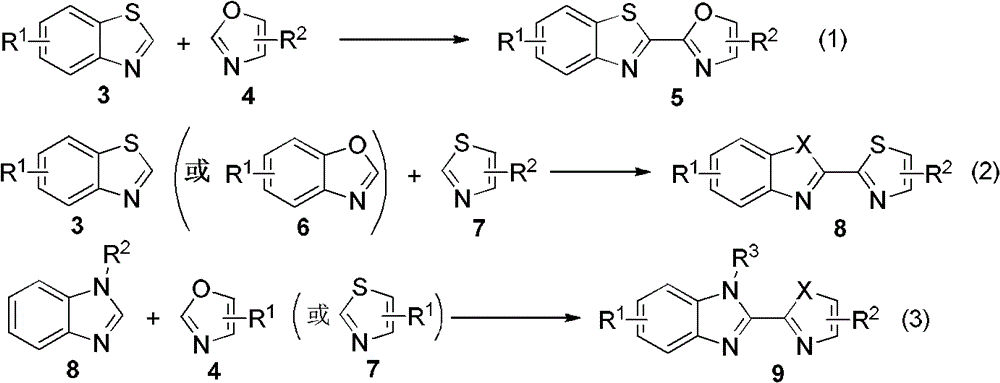
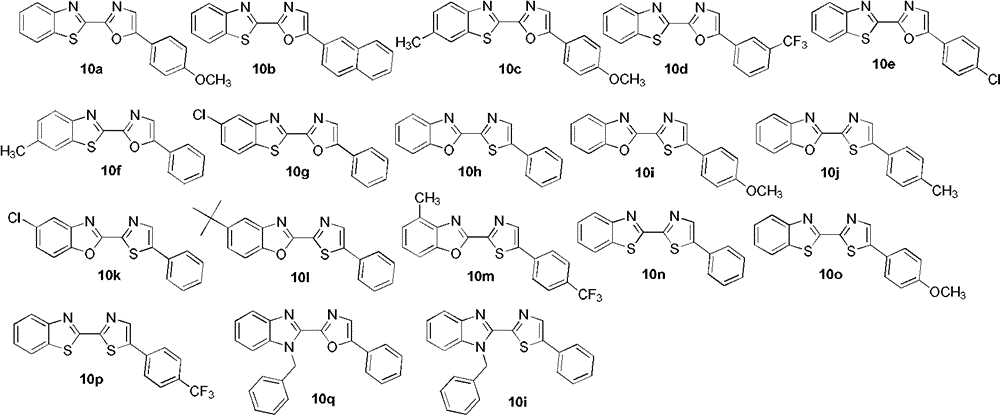
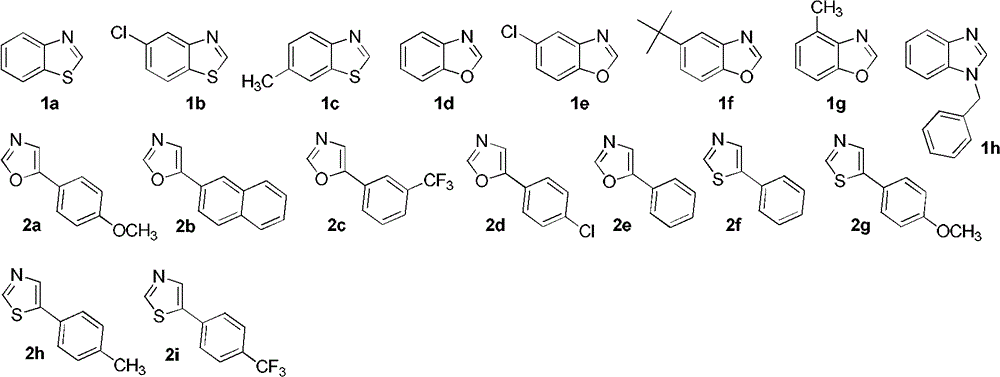
[0028] In a 25mL reaction flask, add AgF (5mg, 0.04mmol), Cu(OAc) 2 (73mg, 0.4mmol), CsF (91mg, 0.6mmol), 2a (53mg, 0.3mmol), after replacing the reaction gas atmosphere with oxygen, add 1a (27mg, 0.2mmol) and 2 ml of DMSO, and stir at room temperature for 2 minutes Afterwards, it was placed in an oil bath at 130°C to start the reaction, and the reaction was tracked by TLC. After the reaction was completed (about 24h), silica gel column chromatography was carried out for separation and purification, and the eluent was petroleum ether (60-90C) / ethyl acetate / triethylamine (v / v / v, 100:10:1) to obtain the target Product 10a (49 mg, 80% yield).
[0029] Formulate compound 10a into 5 × 10 -6 moles / liter of chloroform solution. This solution emits a dazzling blue luster under 365nm ultraviolet light irradiation and has fluorescence activity.
[0030]Referring to the ATCC cell culture method, in DMEM (Dublecco's Minimum Essential Medium) medium (containing 1% dimethylmethylene Le...
Embodiment 2
[0032] The reaction steps were the same as in Example 1, except that 2b (59 mg, 0.3 mmol) was used instead of 2a to obtain the target product 10b (41 mg, yield 63%).
Embodiment 3
[0034] The reaction steps were the same as in Example 1, except that 2c (64 mg, 0.3 mmol) was used instead of 2a to obtain the target product 10c (62 mg, yield 89%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com