Method for purifying ropinirole hydrochloride
A technology of ropinirole hydrochloride and a purification method is applied in the purification field of removing ropinirole hydrochloride oxidized impurities, and can solve the problems of troublesome post-processing, high cost, low content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
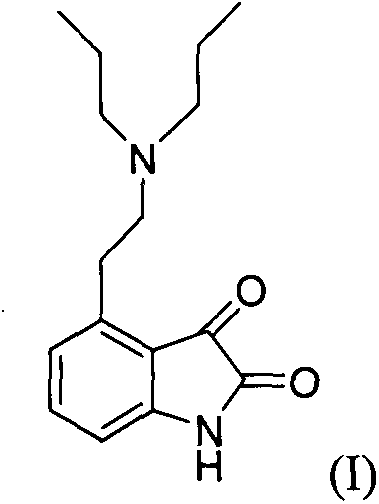
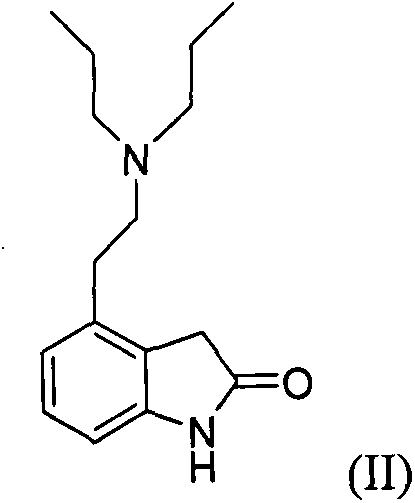
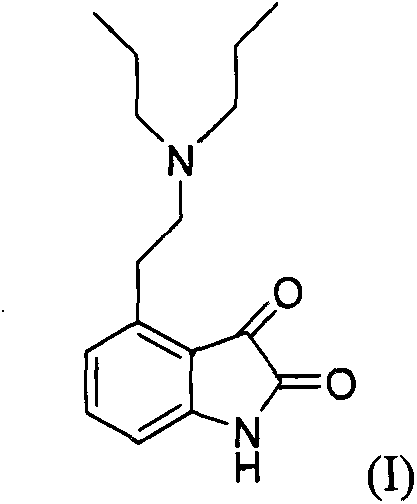
[0021] Get 4-2-di-n-propylamine ethyl-1,3-dihydro-2H-indol-2-one hydrochloride (ropinirole hydrochloride, structural formula II) (6.53g, 0.022mol), toluene 65ml and 100ml of purified water were stirred in a 250ml flask. Stirring was continued and 10.7 g of sodium bicarbonate was slowly added. Continue to stir for 20 min after the addition, and let stand to separate layers. The water layer was removed, and the organic layer was dried with 2.5 g of anhydrous magnesium sulfate, and 0.6 g of activated carbon was added during the drying process, and stirring was continued for 30 min. The organic layer was filtered and concentrated to dryness to give an oil.
[0022] Add 90ml of isopropanol to the obtained oil, control the temperature of the reaction solution at 15±5°C, slowly add 2.2g of concentrated hydrochloric acid, and continue stirring for 30min after the addition is complete. Finally, cool the feed liquid to 5±5°C and continue to stir for 1 hour, and shake it to dryness. ...
Embodiment 2
[0037] Get 4-2-di-n-propylamine ethyl-1,3-dihydro-2H-indol-2-one hydrochloride (ropinirole hydrochloride, structural formula II) (6.53g, 0.022mol), n-hexane 70ml of alkanes and 110ml of purified water were stirred in a 250ml flask. Stirring was continued and 4.0 g of sodium ethoxide was slowly added. Continue to stir for 20 min after the addition, and let stand to separate layers. The water layer was removed, and the organic layer was dried with 2.5 g of anhydrous magnesium sulfate, and 0.8 g of activated carbon was added during the drying process, and stirring was continued for 30 min. The organic layer was filtered and concentrated to dryness to give an oil.
[0038] Add 60ml of isopropanol to the obtained oil, control the temperature of the reaction solution at 15±5°C, slowly add 2.2g of concentrated hydrochloric acid, and continue stirring for 30min after the addition is complete. Finally, cool the feed liquid to 5±5°C and continue to stir for 1 hour, and shake it to dr...
Embodiment 3
[0041] Get 4-2-di-n-propylamine ethyl-1,3-dihydro-2H-indol-2-one hydrochloride (ropinirole hydrochloride, structural formula II) (6.53g, 0.022mol), two 65ml of methyl chloride and 120ml of purified water were stirred in a 250ml flask. Stirring was continued and 5.8 g of sodium hydroxide was slowly added. Continue to stir for 20 min after the addition, and let stand to separate layers. The water layer was removed, and the organic layer was dried with 2.5 g of anhydrous magnesium sulfate, and 0.9 g of activated carbon was added during the drying process, and stirring was continued for 30 min. The organic layer was filtered and concentrated to dryness to give an oil.
[0042] Add 70ml of isopropanol to the obtained oil, control the temperature of the reaction solution at 15±5°C, slowly add 2.2g of concentrated hydrochloric acid, and continue stirring for 30min after the addition is complete. Finally, cool the feed liquid to 5±5°C and continue to stir for 1 hour, and shake it t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com