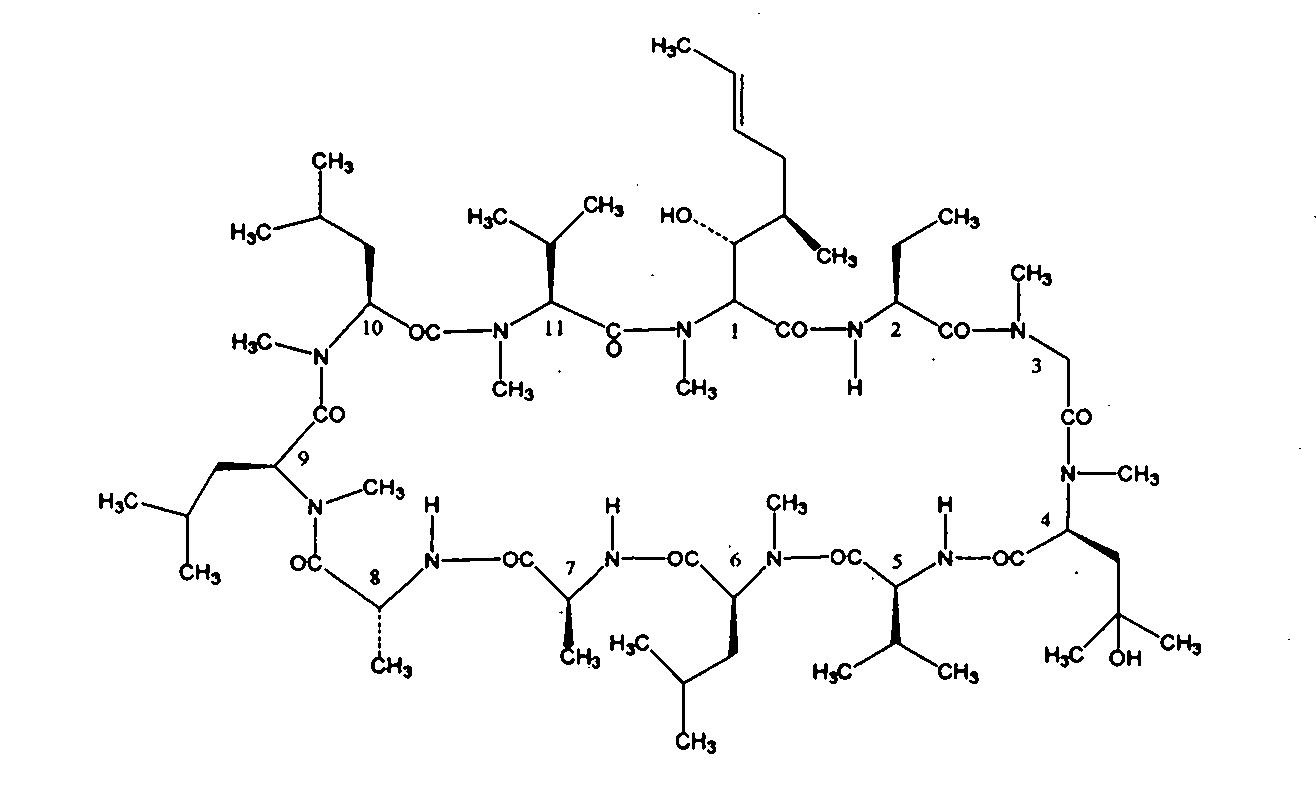
Preparation method of high-purity cyclosporin A derivative
A technology for cyclosporin and derivatives, which is applied in the preparation of pharmaceutical raw materials, separation and purification of cyclosporin A derivatives, and achieves the effects of high sample yield, improved filtration speed, and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
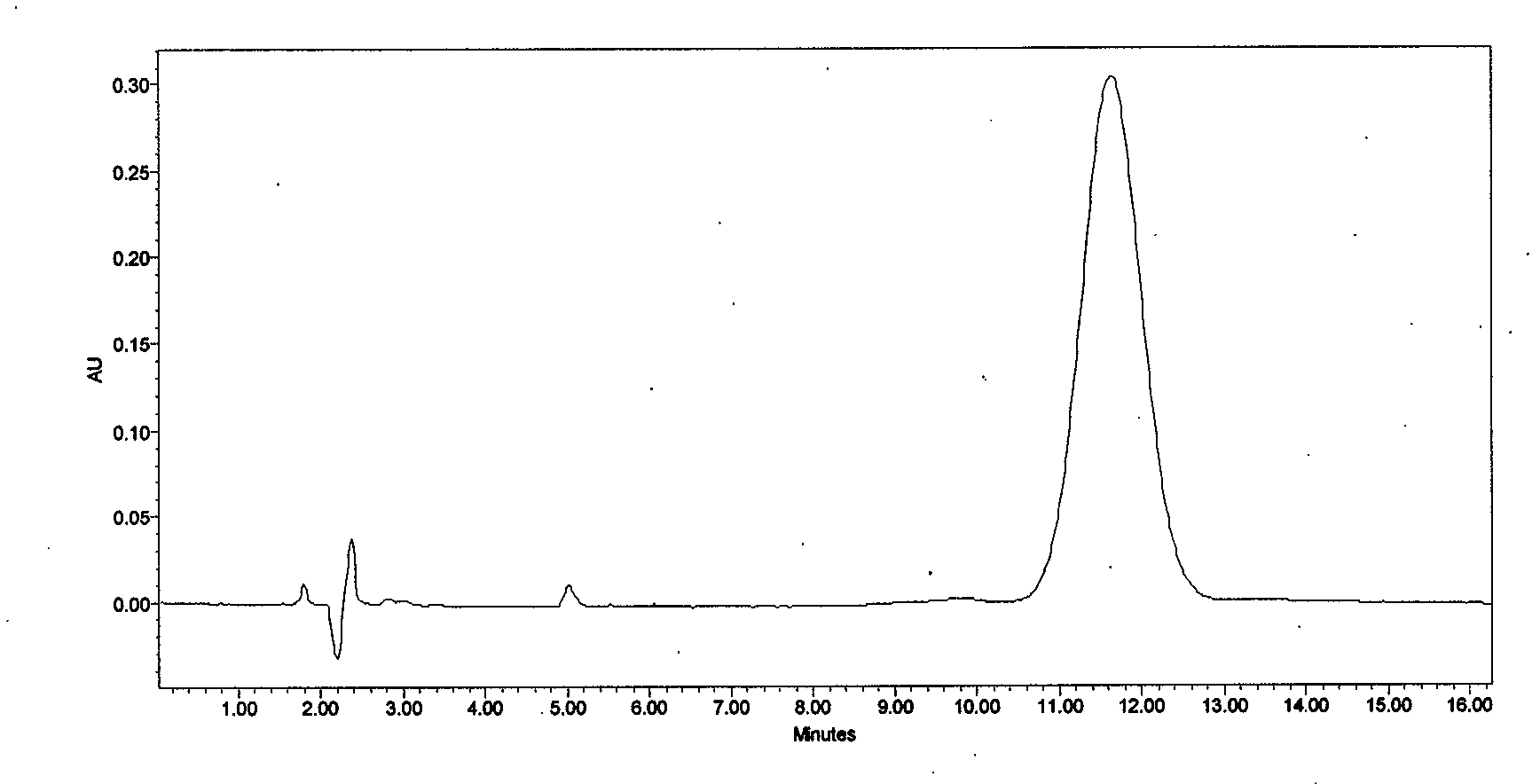
[0037] Take 10L of cyclosporine A derivative fermentation liquid, and the fermentation unit is 422 μg / mL. Add 200g of perlite to the fermentation broth, stir for 50 minutes, and then filter the plate and frame. The filtrate passes through the macroporous resin SD-2 column at a flow rate of 1-2BV / h for decolorization, and the decolorization solution is introduced into the macroporous resin D312 at a flow rate of 1BV / h. The column is subjected to adsorption and enrichment. After adsorption, use 40% ethanol / water solution and 95% ethanol / water solution for discontinuous gradient desorption. The desorption flow rate is controlled at 0.5BV / h. When HPLC detects that cyclosporine A derivatives flow out, start to collect and desorb After the desorption is completed, the desorption liquid is vacuum concentrated to a concentration of cyclosporine A derivatives of 100g / L-120g / L, slowly cooled to -5-0°C, crystallized for 12 hours, filtered, washed, and dried to obtain a coarse powder (see...
Embodiment 2
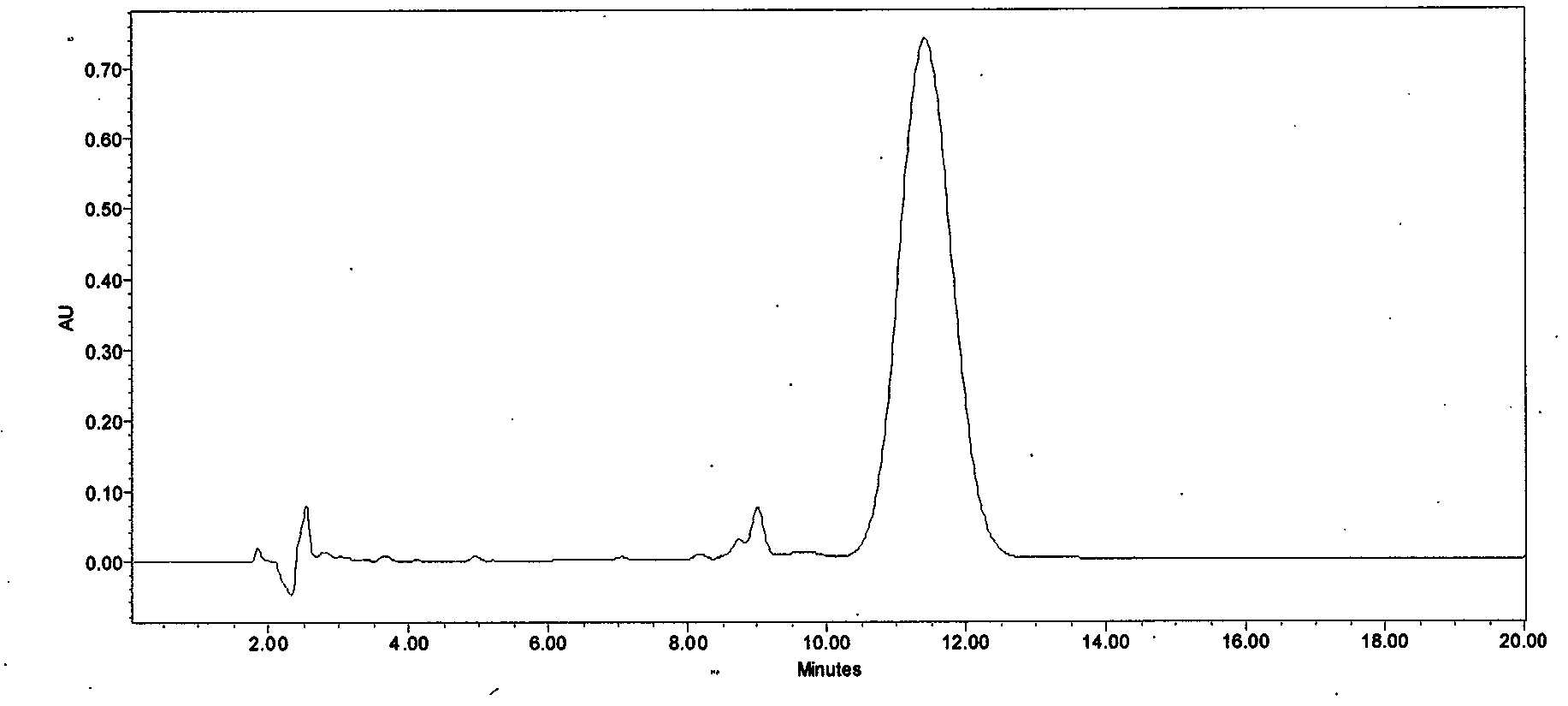
[0039] Take 100 L of cyclosporin A derivative fermentation liquid, and the fermentation unit is 457 μg / mL. Add 3Kg of diatomaceous earth to the fermentation broth, stir for 90 minutes, and filter the plate frame. The filtrate passes through the macroporous resin LX-700 column at a flow rate of 1.5BV / h for decolorization, and the decolorization solution is introduced into the macroporous resin at a flow rate of 1.5BV / h. HZ816 column is used for adsorption and enrichment. After adsorption, use 40% ethanol / water solution and 95% ethanol / water solution for discontinuous gradient desorption. The desorption flow rate is controlled at 0.5BV / h. When HPLC detects that cyclosporin A derivatives flow out, they start to collect Desorption liquid, after the desorption is completed, vacuum concentrate the desorption liquid to the concentration of cyclosporin A derivatives 110g / L-120g / L, slowly cool down to -5-0°C, crystallize for 12 hours, suction filter, wash and dry to obtain crude Powder...
Embodiment 3
[0041] Take 1000L of cyclosporin A derivative fermentation liquid, and the fermentation unit is 428 μg / mL. Add 40Kg of perlite to the fermentation broth, stir for 90 minutes, and then filter the plate and frame. The filtrate passes through the macroporous resin SD-2 column at a flow rate of 1.5BV / h for decolorization, and the decolorization solution is introduced into the macroporous resin HZ816 at a flow rate of 1.5BV / h. The column is subjected to adsorption and enrichment. After adsorption, use 40% ethanol / water solution and 95% ethanol / water solution for discontinuous gradient desorption. The desorption flow rate is controlled at 0.5BV / h-1BV / h. HPLC detects that cyclosporin A derivatives flow out After the desorption is completed, the desorption liquid is concentrated in vacuum to the concentration of cyclosporin A derivatives of 110g / L-120g / L, slowly cooled to -5-0°C, crystallized for 12 hours, suction filtered, washed and dried , to obtain 638.1 g of coarse powder. Add 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com