Probe used for detecting acetylcholin esterase and its inhibitor activity, application and preparation method
A technology for acetylcholinesterase and inhibitors, which is applied in the field of probes for detecting the activity of acetylcholinesterase and its inhibitors, can solve the problems of background interference and low sensitivity, and achieve the effects of high sensitivity, fast response speed and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Formula I Probe Precursor and Vesicle Probe
[0039] 1. Preparation of Formula I Probe Precursor
[0040] In the first step, 0.375g (1mmol) of 10,12-diyne pentapentacic acid was dissolved in 20ml of dichloromethane, and then 2ml of thionyl chloride was added dropwise thereto to obtain a reaction solution, and the reaction was continuously stirred under nitrogen protection solution until the reaction is complete (usually stirred overnight); then, the solvent is distilled off under reduced pressure to obtain the compound of formula II as a colorless oil.
[0041] In the second step, the compound of formula II obtained in the first step is dissolved in a small amount of tetrahydrofuran to obtain a solution of the compound of formula II, and then the solution of the compound of formula II is added dropwise to 20 ml of tetrahydrofuran solution containing 0.18 g of triethylene glycol to obtain a reaction solution. The reaction solution was continuous...
Embodiment 2
[0049] Example 2 Spectral properties and color changes of the vesicle probe reacting with myristoylcholine chloride
[0050] 1. Spectral properties of the reaction of vesicle probes with myristoylcholine chloride
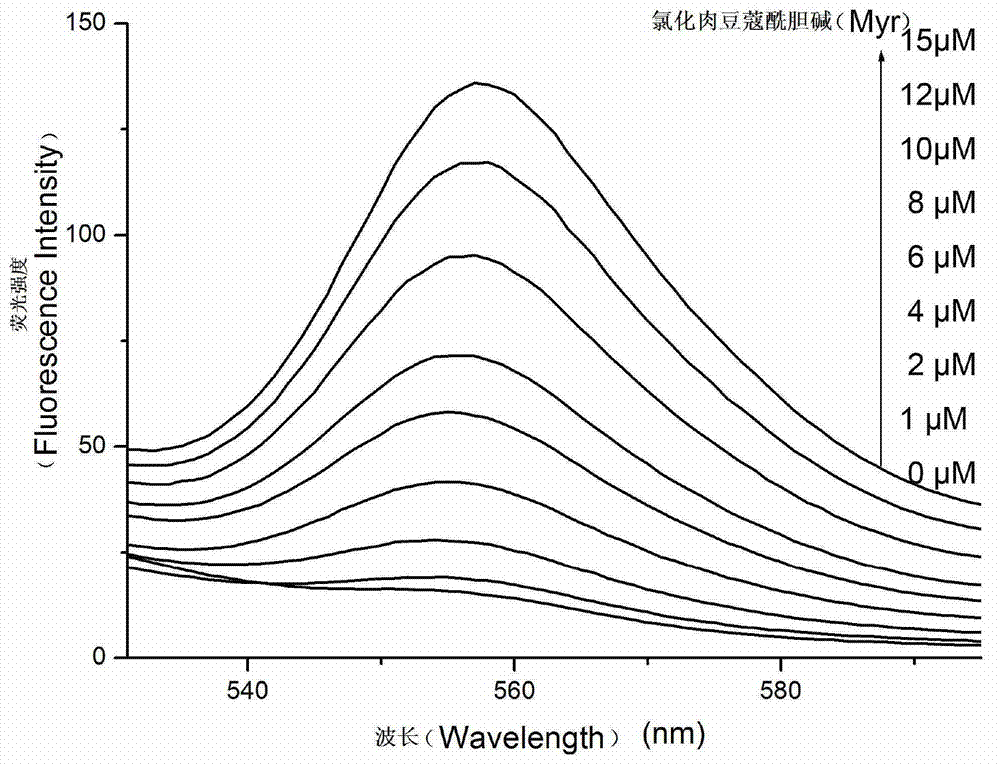
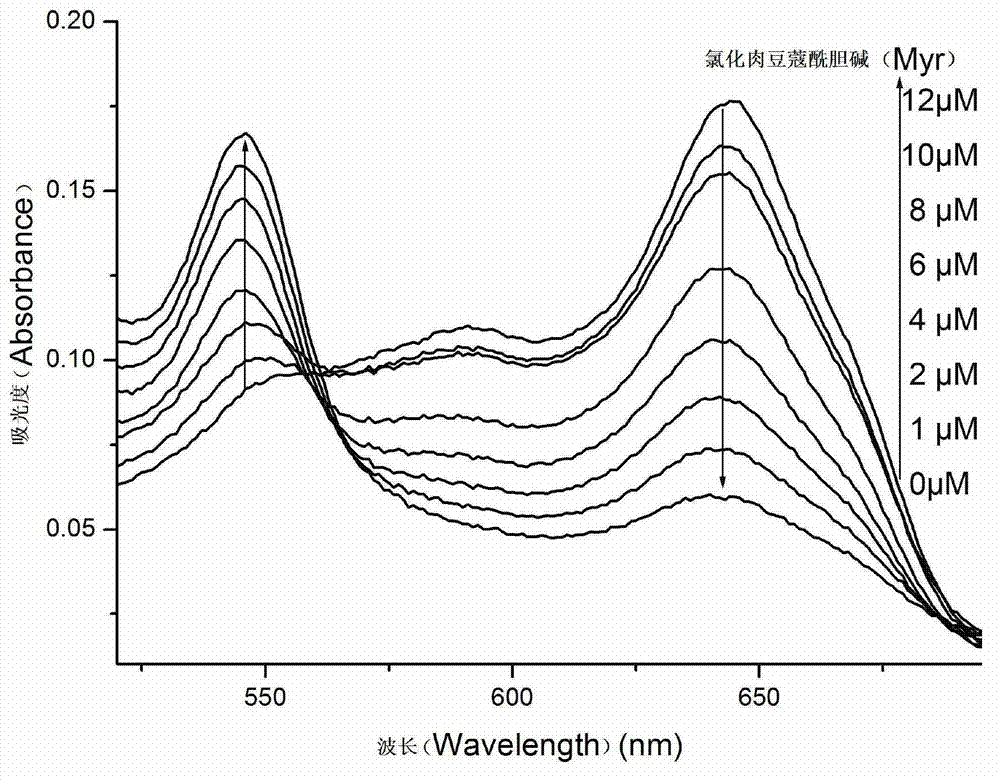
[0051] Take nine 60μl 1mM vesicle probes and add them to each quartz dish, then add 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer (10mM pH=7.4) to dilute and dilute to 3ml; then , add 0 μl, 0.6 μl, 1.2 μl, 1.8 μl, 2.4 μl, 3 μl, 3.6 μl, 4.5 μl of 10 mM myristoylcholine chloride (final concentration of 0 μM, 2 μM, 4 μM, 6 μM, 8 μM, 10 μM, 12 μM , 15 μM), the fluorescence emission spectrum and UV-vis absorption spectrum were measured after reacting for 5 minutes. The fluorescence emission spectrum was measured with excitation at 492nm, and the slit width between excitation and emission was 5nm.
[0052] Fluorescence emission spectra such as figure 1 As shown, when the concentration of myristoylcholine chloride is in the range of 0-15 μM, the fluorescenc...
Embodiment 3
[0057] Example 3 Spectral properties and color changes of the reaction product of myristoylcholine chloride and acetylcholinesterase (AChE) mixed with vesicle probes
[0058] 1. Spectral properties of the reaction product of myristoylcholine chloride and acetylcholinesterase (AChE) mixed with vesicle probes
[0059] Take seven 3.6μl 10mM myristoylcholine chloride and add to each quartz dish, add 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer (10mM pH=7.4) to dilute and finally make up to 3ml ; Then, add an appropriate amount (such as 2 μl) of different concentrations of acetylcholinesterase (AChE) (0U / ml, 0.025U / ml, 0.05U / ml, 0.1U / ml, 0.2U / ml, 0.3U / ml, 0.4 U / ml); after 10 minutes of reaction, 60 μl of 1mM vesicle probes were added to the quartz dish, and then reacted for 5 minutes, and finally the fluorescence emission spectrum and ultraviolet-visible light absorption spectrum were measured. The fluorescence emission spectrum was measured with excitation at 492nm,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com