Supported nickel-based catalyst and its preparation method and use
A nickel-based catalyst, supported technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. It is difficult to remove catalysts and other problems to achieve the effect of slowing down deactivation, improving conversion rate, and achieving significant effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
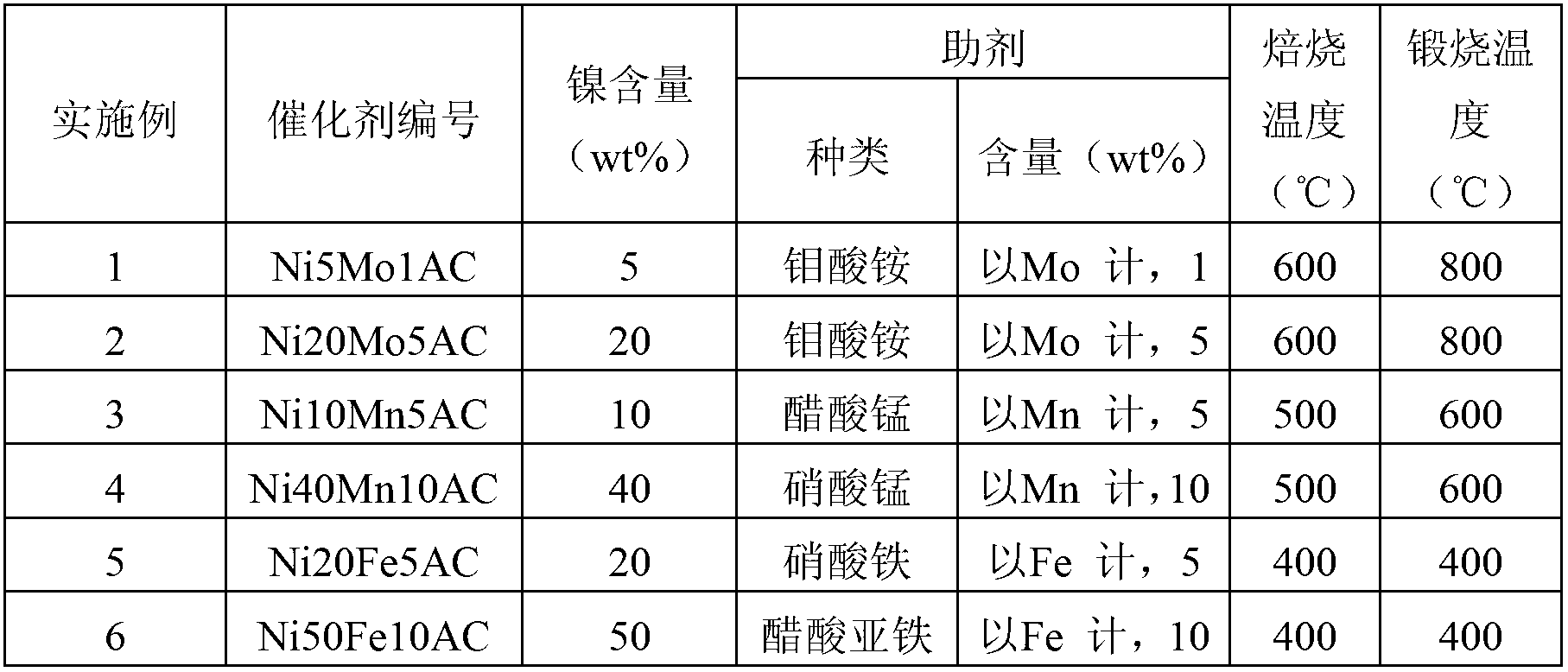
Embodiment 1-6
[0039] Preparation of activated carbon-supported nickel-based catalysts by impregnation method:
[0040] Pretreatment of the carrier: 1g of activated carbon (110-130 mesh, specific surface area 770m 2 ﹒ g -1 ) in a nitrogen atmosphere at 600°C for 6 hours to obtain treated activated carbon;
[0041] Precursor preparation: impregnate the treated activated carbon in 20ml of a mixed aqueous solution containing nickel nitrate and ammonium molybdate additives, wherein the mass of nickel in nickel nitrate is 5% of the mass of activated carbon (that is, nickel content), ammonium molybdate The mass of molybdenum in the medium is 1% of the mass of the activated carbon, and it is impregnated at 20 °C for 8 hours to prepare the Ni-Mo / AC catalyst precursor;
[0042] Catalyst reduction: The Ni-Mo / AC catalyst precursor was vacuum dried at 120°C for 6h, calcined at 800°C for 6h in a nitrogen atmosphere, and then reduced with hydrogen at 600°C for 10h to finally obtain the desired activate...
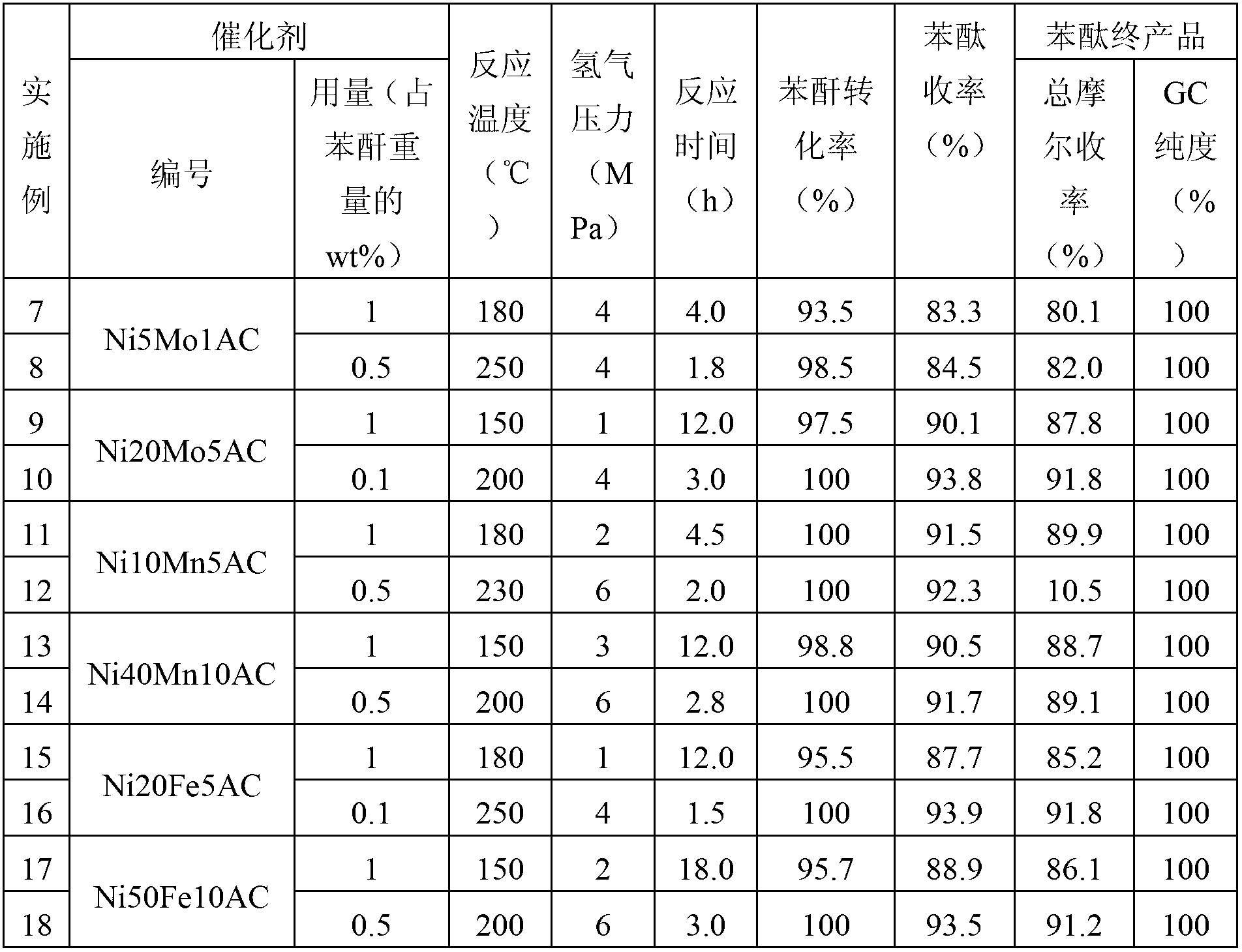
Embodiment 7-18
[0047] To evaluate the activity of the synthesized catalyst, the steps are as follows:
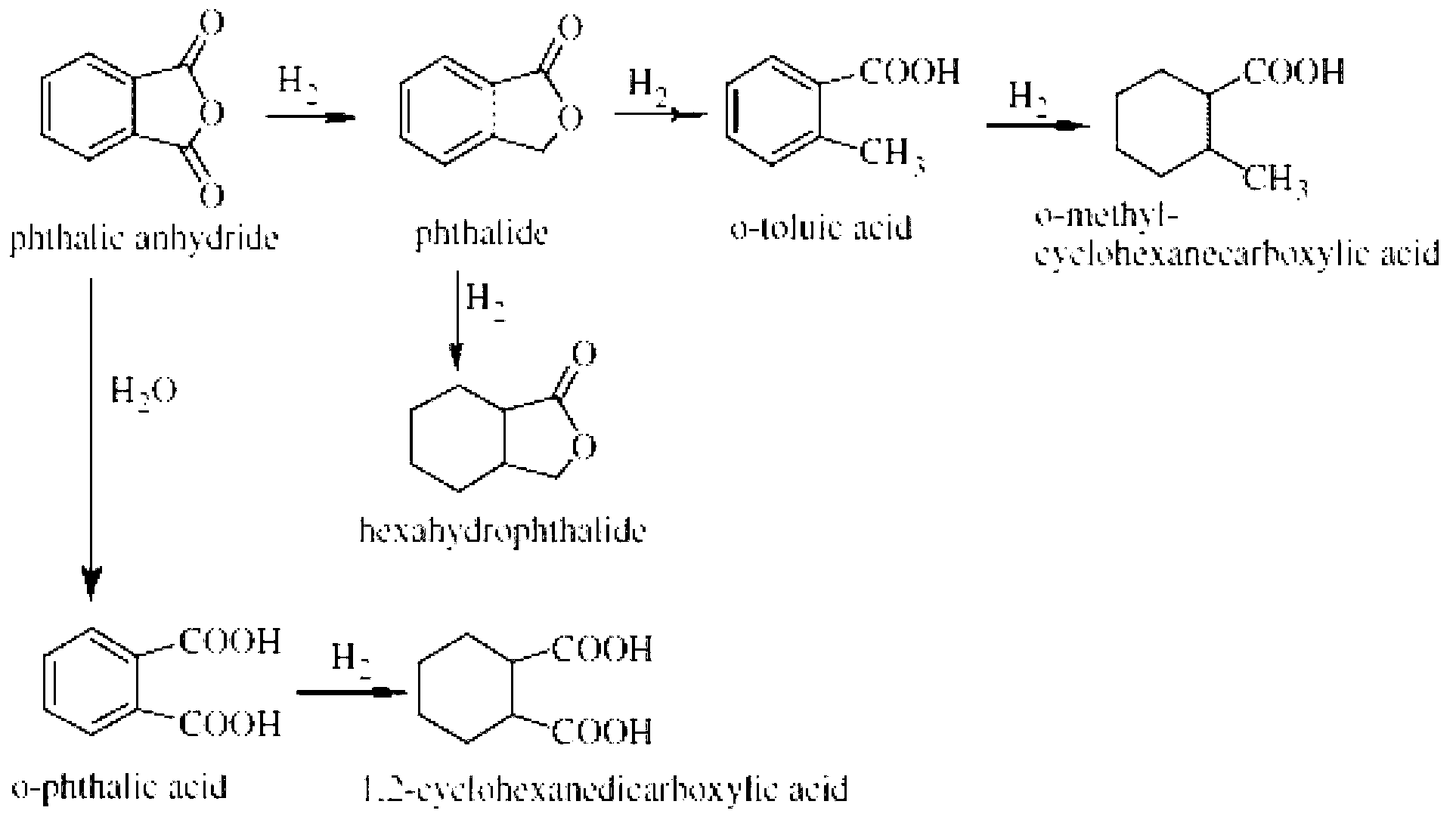
[0048] According to the mass ratio of phthalic anhydride: phthalide (reaction solvent): Ni5Mo1AC catalyst is 20g: 40g: 0.2g, add 100ml of high-pressure stirring reaction kettle in turn, replace the air with 1MPa nitrogen three times, then raise the temperature to 180°C, start stirring, and feed The hydrogen pressure was maintained at 4.0 MPa, and the reaction was terminated after 4 hours. After the reaction, let it cool down to 120°C, open the kettle, and heat filter to recover the catalyst. It was detected that the conversion rate of phthalic anhydride was 93.5%, and the yield of phthalide was 83.3%. The product was washed with 10% sodium carbonate solution and filtered. Then vacuum rectify the product at -0.09MPa (column size 3cm×70cm, triangular pyramid stainless steel packing), collect 40-80°C fraction; finally increase the vacuum to -0.097MPa, and further distill out the low boiling ...
Embodiment 19-21
[0055] The service life of the synthesized Ni-Mo / AC, Ni-Mn / AC and Ni-Fe / AC catalysts was evaluated in a high-pressure stirred reactor, and the steps were as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com