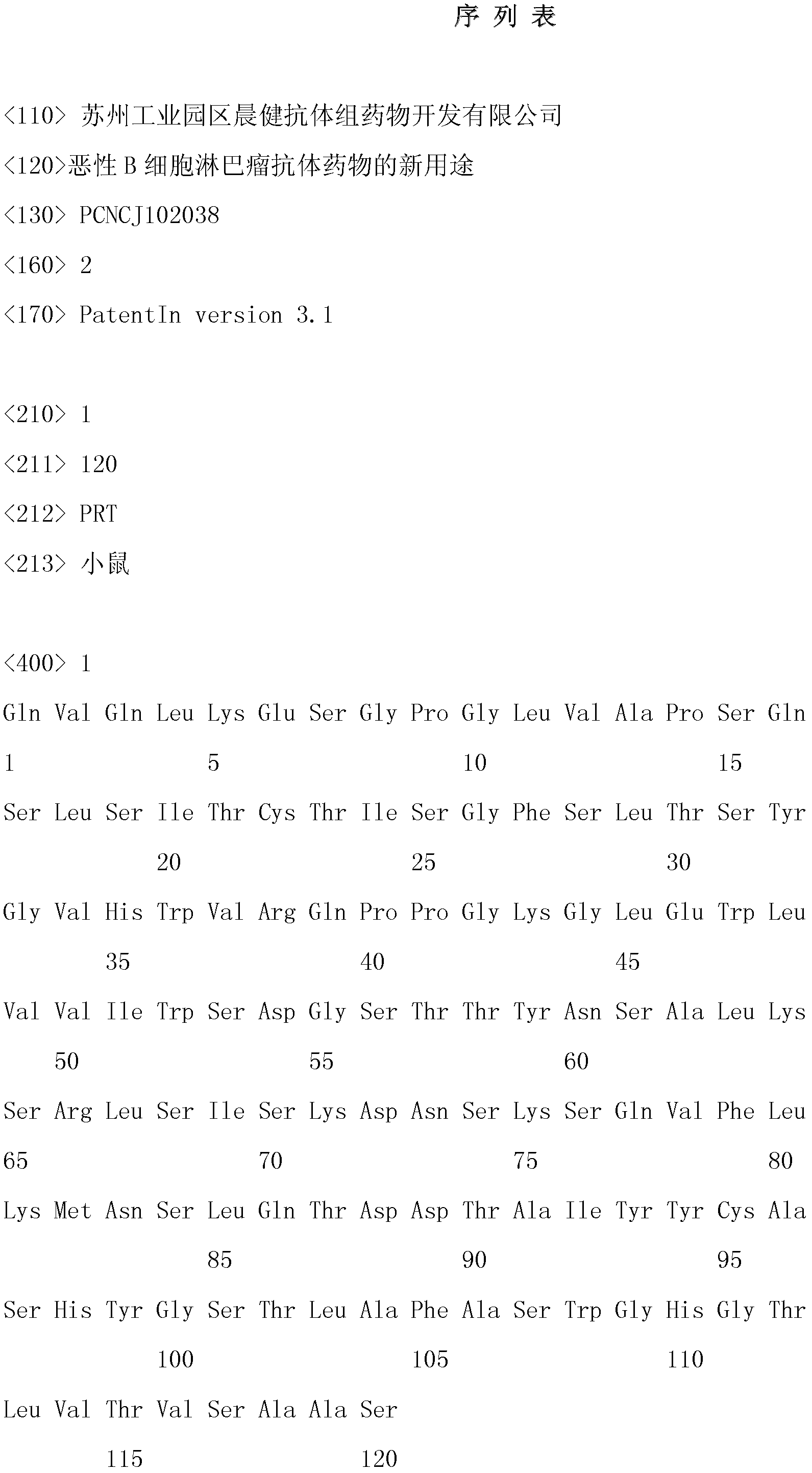New application of malignant B-cell lymphoma antibody medicament
A lymphoma-specific technology, applied in the direction of antibodies, drug combinations, anti-tumor drugs, etc., can solve the problems of easy recurrence, uncertain effect on relapsed patients, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of lymphoma-specific humanized antibody against HLA-DR10
[0027]Using the mouse myeloma cell line NSO-clym with the preservation number CCTCC No. 200405 recorded in the Chinese patent (patent number: 200410053872.0), inoculate the cell line in SFM containing 10% serum and not containing L-glutamine culture medium at 37°C, 5% CO 2 Cultivate in an incubator, collect the culture supernatant, and load the filtered cell-free supernatant onto the FineLINE column filled with MabSelect at a concentration of 30 mg / ml. Immediately after elution, the eluate was neutralized to pH 6.8-7.0 with potassium phosphate. Then it was detected by gel filtration, and the result showed that the purity was above 95%. The product was sequenced, and the result was that the amino acid sequence of the heavy chain variable region of the antibody was SEQ ID NO: 1, and the amino acid sequence of the light chain variable region was SEQ ID NO: 2, which met expectations. The met...
Embodiment 2
[0028] Example 2 131 Preparation of I-labeled lymphoma-specific humanized antibody against HLA-DR10
[0029] Using chloramine-T iodide labeling method, the 131 I was labeled on the lymphoma-specific humanized antibody against HLA-DR10 obtained in Example 1, and the radioactive specific activity of the product was 11 mCi / mg. The affinity constant Ka=3.40×10 of the monoclonal antibody was detected by the method of Example 1. 8 mol -1 .
Embodiment 3
[0030] Example 3 Pharmacodynamic study of lymphoma-specific humanized antibody against HLA-DR10 in the treatment of leukemia
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com