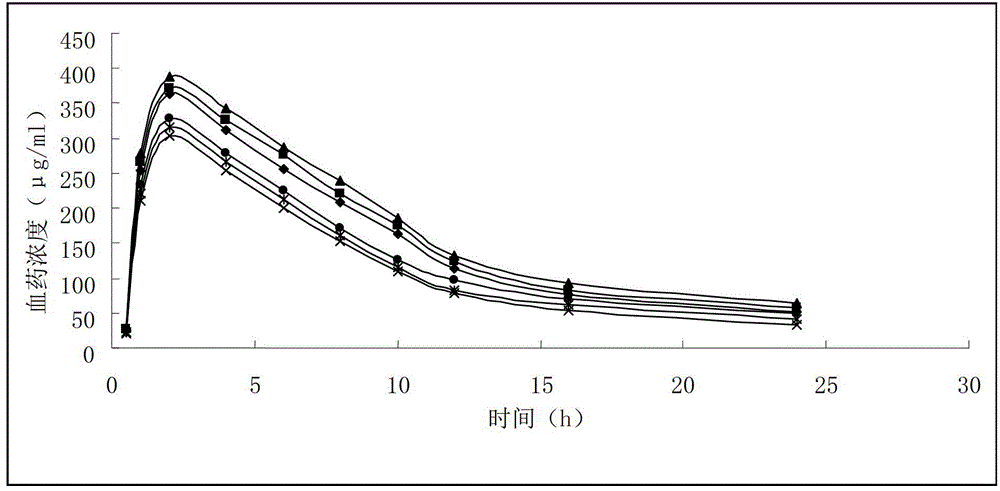
Xiyanping lipidosome injection
A technology for injections and liposomes, applied in the field of medicine, can solve the problems of easy aggregation, fusion, and leakage of encapsulated drugs in liposomes, and achieve the effects of improving bioavailability, low leakage rate, and improving the quality of preparation products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] On the other hand, the present invention also provides a preparation method of Xiyanping liposome injection, specifically comprising the following preparation steps:
[0057] (1) Dissolve phosphatidylcholine distearate, soybean phosphatidylinositol, soybean sterol and sodium deoxycholate in an organic solvent under slow magnetic stirring at about 55°C to obtain a lipid solution, and dissolve the above solution Freeze-drying until the drying is complete to obtain a dry solid that forms a loose spongy shape;
[0058] (2) Dissolve Xiyanping in phosphate buffer solution, add povidone, and stir evenly to obtain an aqueous phase;
[0059] (3) Under slow magnetic stirring at about 45°C, add the solid in step 1 into the water phase in step 2, then perform gradient homogenization at 500bar to 700bar for 7 to 9 times, and filter with a 0.22μm microporous membrane to obtain Xiyanping liposome;
[0060] (4) Under the protection of nitrogen, adjust the pH value of the Xiyanping li...
Embodiment 1
[0075] The preparation of embodiment 1 Xiyanping liposome injection
[0076] Prescription: (100 sticks)
[0077] The components and weights of the raw and auxiliary materials used are as follows:
[0078]
[0079]
[0080] Prepare Xiyanping liposome injection by preparation process:
[0081] (1) Dissolve 300g of phosphatidylcholine distearate, 150g of soybean phosphatidylinositol, 100g of soybean sterol and 50g of sodium deoxycholate in 900mL of The lipid solution was obtained in a mixed organic solvent of tert-butanol and methanol, and the above solution was pre-frozen at -60°C for 2 hours, then frozen at -40°C for 16 hours, then sublimated to 20°C for 12 hours, and finally dried at 30°C After 3 hours, a loose spongy dry solid was obtained;
[0082] (2) Dissolve 25g of Xiyanping in 10,000ml of phosphate buffer solution with a pH of 7.8, add 75g of povidone, and stir evenly to obtain an aqueous phase;
[0083] (3) Under slow magnetic stirring at about 45°C, add the...
Embodiment 2
[0085] The preparation of embodiment 2 Xiyanping liposome injection
[0086] Prescription: (100 sticks)
[0087] The components and weights of the raw and auxiliary materials used are as follows:
[0088]
[0089]
[0090] Prepare Xiyanping liposome injection by preparation process:
[0091] (1) Dissolve 125g of phosphatidylcholine distearate, 50g of soybean phosphatidylinositol, 37.5g of soybean sterol and 12.5g of sodium deoxycholate in 300mL under slow magnetic stirring at about 55°C with a volume ratio of 2: 1 in a mixed organic solvent of tert-butanol and methanol to obtain a lipid solution. The above solution was pre-frozen at -70°C for 4 hours, then frozen at -50°C for 18 hours, then sublimated to 25°C for 14 hours, and finally at 35°C. ℃ and dried for 5 hours to obtain a loose spongy dry solid;
[0092] (2) Dissolve 12.5g of Xiyanping in 5000ml of phosphate buffer solution with a pH of 7.8, add 25g of povidone, and stir evenly to obtain an aqueous phase;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com