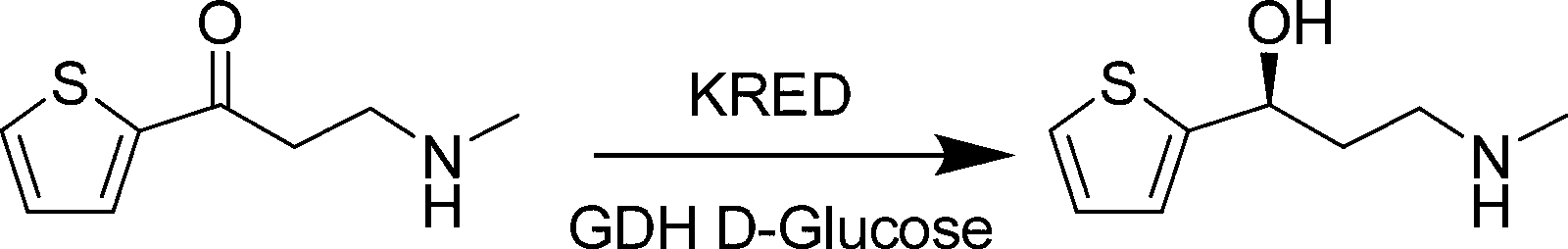
Biological preparation method of (S)-3-methylamino-(2-thienyl)-1-propyl alcohol
A biological preparation and thiophene-based technology, which is applied in the field of biopharmaceuticals and green chemistry, can solve the problems of difficult industrialization, high catalyst addition, and low substrate concentration, so as to increase substrate concentration, reduce enzyme dosage, and reduce production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 1.0 g of substrate MMAK and 1.2 g of glucose into a 50 mL three-necked reaction flask, and then add 8 mL of prepared 0.1 M triethanolamine buffer solution with a pH of 7.0 to the reaction flask. Stir in a 900r / min magnetic stirring water bath at 30°C, and adjust the pH to 7.0 with 2M NaOH solution. When the temperature is stable, add 0.95 mL of triethanolamine buffer in which 20 mg of ketoreductase and 31 mg of GDH are dissolved in the reaction flask. Adjust the pH to 7.0 with NaOH and rinse with 0.95 mL of triethanolamine buffer and add to the reaction flask. Add 100 μL of triethanolamine buffer dissolved in 0.5 mg NADP to the reaction flask, and the reaction starts. The reaction temperature was maintained at 30°C. The pH of the reaction solution was adjusted by dropping 2M NaOH solution with a pH titrator. The pH is controlled at 7.0, the initial control point is 6.5, and the final control point is 6.99. Regular sampling for HPLC detection central control. Af...
Embodiment 2
[0023] Add 100.0 g of substrate MMAK and 120.0 g of glucose into the reactor, and then add 800 mL of prepared pH 7.0, 0.1 M triethanolamine buffer into the reaction flask. Place at 30°C, stir in a mechanically stirred water bath, and adjust the pH to 7.0 with 2M NaOH solution. When the temperature is stable, add 95 mL of triethanolamine buffer dissolved in 2 g of ketoreductase and 3.1 g of GDH into the reaction flask. Adjust the pH to 7.0 with NaOH and rinse with 95 mL of triethanolamine buffer and add to the reaction flask. Add 10 mL of triethanolamine buffer solution that is dissolved with 50 mg NADP in the reaction bottle again, and the reaction starts. The reaction temperature was maintained at 30°C. The pH of the reaction solution was adjusted by dropping 2M NaOH solution with a pH titrator. The pH is controlled at 7.0, the initial control point is 6.5, and the final control point is 6.99. Regular sampling for HPLC detection central control. After 23 hours of reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com