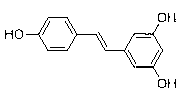
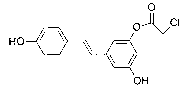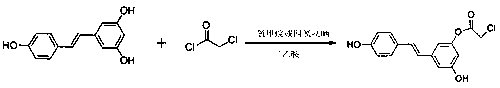(E)-3-hydroxy-5-(hydroxystyryl)-2-phenyl chloroacetate compound and preparation method thereof
A technology of hydroxystyrene and phenyl chloroacetate, which is applied in the preparation of carboxylic acid halides, digestive systems, organic chemistry, etc., can solve the problems of easy oxidation and instability, and achieve stable structure, convenient operation, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of (E)-3-hydroxyl-5-(hydroxystyryl)-2-chlorophenylacetate compound, specifically comprising the following steps:
[0032] (1) Add 114mg (2.2mmol) resveratrol into a 100mL three-necked flask, and stir with 20mL dichloromethane for 5-15min to obtain a white turbid liquid;
[0033] Add 1 mL of triethylamine to the white turbid solution obtained above in an ice bath, and stir for 2 to 5 minutes to obtain a yellow clear solution;
[0034] Slowly add 248 mg (2.2 mmol) of chloroacetyl chloride dropwise to the yellow clear liquid obtained above under ice bath conditions, remove the ice bath after the dropwise addition, and raise the temperature to 25°C for esterification for 2 to 5 hours to obtain a reaction liquid;
[0035] (2), control the temperature of the water bath obtained in step (1) to 20-40°C, and the pressure to 0.07-0.1MPa to carry out the first decompression concentration to remove excess dichloromethane and triethylamine in the react...
Embodiment 2
[0039] A preparation method of resveratrol derivatives, specifically comprising the following steps:
[0040] (1) Add 114mg (2.2mmol) resveratrol into a 100mL three-neck flask, stir 20mL tetrahydrofuran for 5-15min to obtain a white clear liquid;
[0041] Add 1 mL of triethylamine to the white clear liquid obtained above in an ice bath, and stir for 2 to 5 minutes to obtain a white turbid liquid;
[0042] Slowly add 248 mg (2.2 mmol) of chloroacetyl chloride dropwise to the white cloudy liquid obtained above under ice bath conditions, remove the ice bath after the dropwise addition, and raise the temperature to 25°C for esterification reaction for 2 to 5 hours to obtain a reaction liquid;
[0043](2) Control the temperature of the water bath obtained in step (1) between 20-40°C and the pressure at 0.07-0.1 MPa to conduct the first vacuum concentration to remove tetrahydrofuran and triethylamine in the reaction liquid, then concentrate The final crude produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com