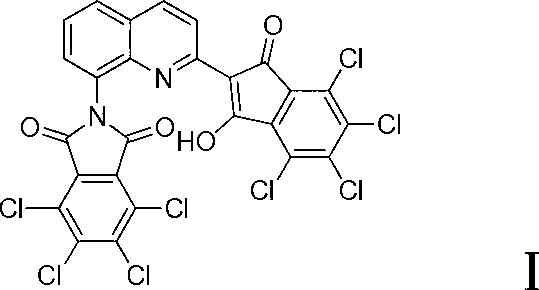
Method for synthesizing quinaphthalone yellow
A quinaldine and mixture technology, which is applied in the field of synthesizing quinophthalone yellow (C.I.P.Y.138), can solve the problems of high loss of target product, harsh reaction conditions, difficult recovery and the like, and achieves clean and safe solvent recovery process, improved yield, The effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
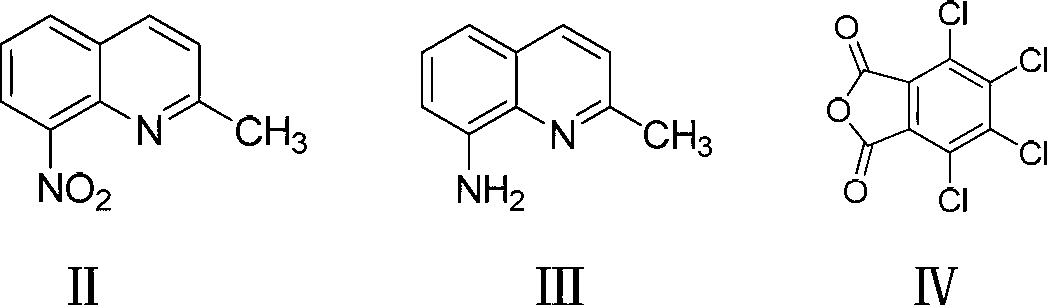
[0028] Add 400ml of tetrahydrofuran, 55g of 8-nitroquinaldine (99.5%), and 0.5g of Pd / C into a small hydrogenation reactor. The airtight reaction device was fully stirred, then the stirring was stopped, and the air in the device was replaced with nitrogen, and the process was repeated three times. Then connect the hydrogen cylinder, replace the nitrogen in the kettle by pressure, and keep the pressure in the device at 0.2MPa-0.3MPa after repeating three times. Slowly start the stirring device at room temperature, gradually increase the speed to 400-500r / min, keep the system temperature at 25°C-40°C through the circulating water device, gradually stop stirring after 5 hours of stirring, and the reduction reaction is completed. The hydrogen in the kettle was replaced with nitrogen, and this was repeated three times. Open the hydrogenation reactor at normal temperature, filter out the clear reaction solution, and collect the reduced 8-aminoquinaldine and the reaction solvent by ...
Embodiment 2
[0031] Add 500ml of methanol into the hydrogenation reaction device, add 50g of 8-nitroquinaldine and 1g of Raney Ni, replace the air in the device with nitrogen, and repeat three times, then replace the nitrogen with hydrogen, and keep the internal pressure of the closed reaction device after repeating three times is 0.5MPa. Stir at room temperature, keep the temperature at 25° C. to 35° C. through a circulating water device, stir for 8 hours, replace the remaining hydrogen in the reaction device with nitrogen, and repeat twice. The clear reaction solution was filtered out, and 8-aminoquinaldine and the reaction solvent were collected by distillation under reduced pressure. The reaction yield was 97.5%, and the purity was 98.0%.
[0032] 1 H NMR (400Hz, CDCl 3 ):δ2.71(s,3H),4.99(s,2H),6.93(d,1H),7.14(d,1H),7.25-7.31(m,2H),7.97(d,1H);IR(pressure sheet method): 3466.78(s,ν-N-H), 3343.71(s,ν-N-H), 3044.23(w,ν=C-H), 2914.05(m,ν-C-H); MS: M + =158.2.
experiment example 3
[0034] Install a thermometer and a vacuum fractional distillation condensing device in a four-neck flask with mechanical stirring. Add 500ml of Dowthem A, 0.6g of pentafluorobenzoic acid, 35.5g of tetrachlorophthalic anhydride and 11g of reduced 8-aminoquinaldine. The temperature was raised to 120°C and stirred for 4 hours, and then the temperature was raised to 180°C for 6 hours. During the addition process, a vacuum fractionation device is connected so that the water generated by the reaction can be discharged from the reaction system in time. The flask was allowed to cool to room temperature and filtered, and the filter cake was washed with methanol until Dowthem A stuck to the pigment was washed away. The first filtered solvent was distilled to recover pentafluorobenzoic acid and Dowthem A. The filtrate of the filter cake was washed a second time with methanol, and methanol and Dowthem A were recovered by distillation. The filter cake was dried and pulverized to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com