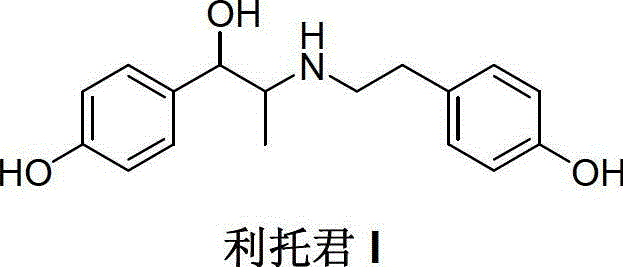
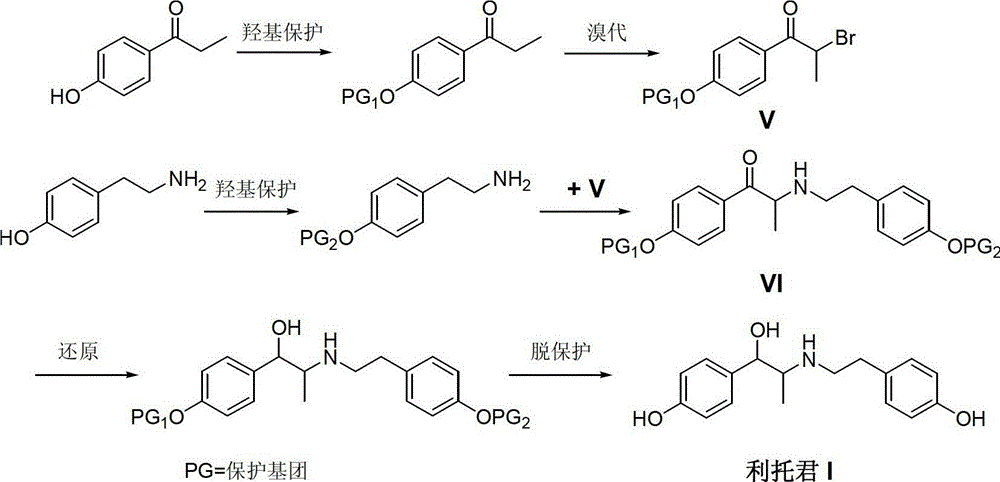
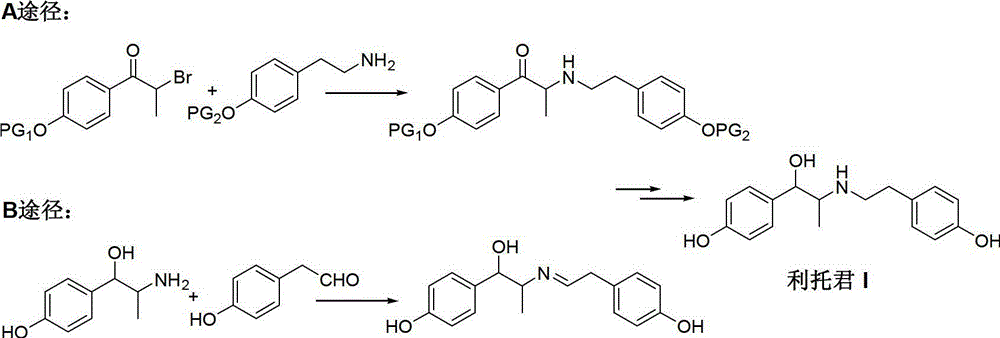
Preparation method of ritodrine
A technology of hydroxyphenyl and intermediates, applied in the field of preparation of ritodrine, to achieve the effect of cost reduction, quality improvement, and promotion of economic and technological development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 4-Hydroxypropiophenone (150 g, 1 mol) and 400 mL of tetrahydrofuran were added to a 1 L three-necked flask, and dry hydrogen chloride gas was introduced under stirring until the system was saturated. Slowly feed methyl nitrite gas (67.0 g, 1.1 mol) at 40° C. for about 4 hours, and continue the reaction for 1 hour after the passage. TLC detects that the reaction is complete. The solvent was recovered under reduced pressure, and the residue was recrystallized from toluene to obtain 139.6 g of off-white solid 2-oximino-4-hydroxypropiophenone (II), with a yield of 78.2%.
Embodiment 2
[0031] Add intermediate 2-oximino-4-hydroxypropiophenone (II) (89.5g, 0.5mol), 10% palladium carbon catalyst (4.5g, 5%w / w), 36% 80mL of concentrated hydrochloric acid and 400mL of methanol, start stirring, according to the operating procedures of catalytic hydrogenation, feed hydrogen to 0.5MPa, heat up to 60-65°C, until no hydrogen is absorbed, continue stirring for one hour. Cool down to room temperature, discharge, and filter to recover the catalyst. Concentrate under reduced pressure to recover methanol. The residue was recrystallized from isopropanol to obtain 56.5 g of off-white solid 2-amino-1-(4-hydroxyphenyl)propanol hydrochloride (III), with a yield of 75.2%.
Embodiment 3
[0033] Add intermediate 2-amino-1-(4-hydroxyphenyl)propanol hydrochloride (III) (75.5g, 0.5mol), triethylamine (10.0g, 0.1mol) and no Water ethanol 250mL, warm up to 50-55°C, stir until the system dissolves uniformly. P-Hydroxyphenylacetaldehyde (68.0 g, 0.5 mol) was slowly added dropwise to the reaction liquid, and the drop was completed in about 1 hour. Keep this temperature and continue to react for 3 hours, and TLC detects that the reaction is complete. Ethanol was recovered under reduced pressure, and the residue was washed with n-hexane to obtain 121.3 g of yellow solid (1-(4-hydroxyphenyl)-2-[2-(4-hydroxyphenyl)ethylimine]propanol (IV) , yield 85.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com