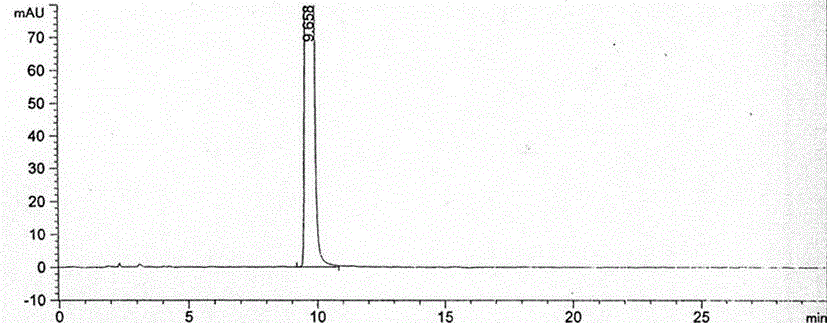
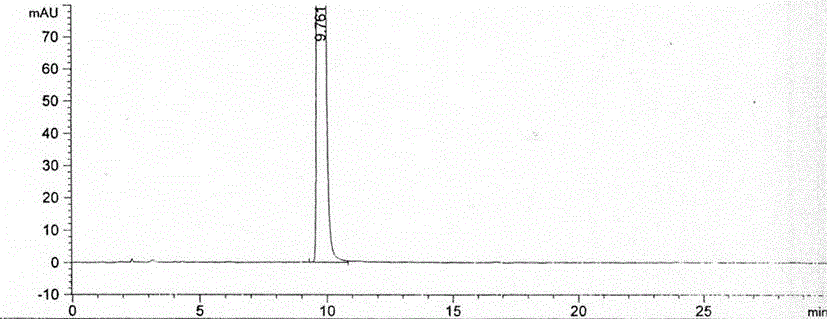
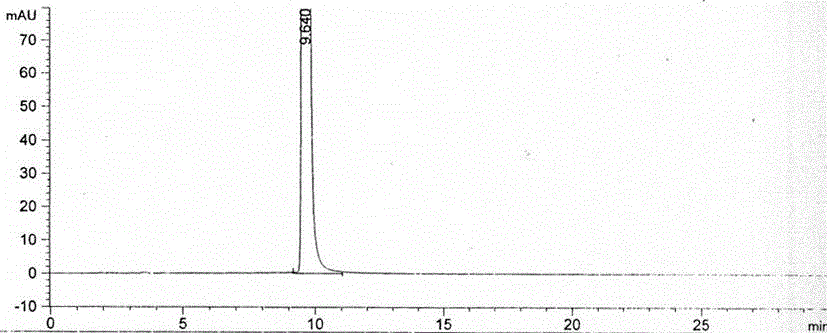
Method for preparing nitisinone
A technology of nitisinone and nitro, which is applied in the preparation of medicines and the field of nitisinone preparation, can solve the problems of large residual toxicity of products, large side effects, high toxicity of cyanide, etc., and achieve reduced toxicity and side effects , The effect of small toxic residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of Nitisinone of the present invention, the detailed steps of this preparation method are as follows:
[0033] The preparation of a, 2-nitro-4-trifluoromethylbenzoyl chloride:
[0034] Taking 2-nitro-4-trifluoromethylbenzoic acid and sulfur oxychloride as raw material, the mol ratio of adding amount between the two of 2-nitro-4-trifluoromethylbenzoic acid and sulfur oxychloride is 1:2, weigh two kinds of raw materials according to the ratio between the two, add the weighed raw materials 2-nitro-4-trifluoromethylbenzoic acid and thionyl chloride into the reactor, heat to 70 ℃ for reflux reaction for 3 hours. After the reaction, the excess thionyl chloride was distilled off, and then the fraction at 158°C was collected under the condition of a vacuum of 0.095mPa. The collected fraction was 2-nitro-4-trifluoromethyl Benzoyl chloride;
[0035] b, the preparation of Nitisinone crude product:
[0036] 2-nitro-4-trifluoromethylbenzoyl chloride, 1,3-cy...
Embodiment 2
[0040] The preparation method of Nitisinone of the present invention, the detailed steps of this preparation method are as follows:
[0041] The preparation of a, 2-nitro-4-trifluoromethylbenzoyl chloride:
[0042] Taking 2-nitro-4-trifluoromethylbenzoic acid and sulfur oxychloride as raw material, the mol ratio of adding amount between the two of 2-nitro-4-trifluoromethylbenzoic acid and sulfur oxychloride is 1:2.5, take two kinds of raw materials according to the ratio between the two, add the weighed raw materials 2-nitro-4-trifluoromethylbenzoic acid and thionyl chloride into the reactor, heat to 75 ℃ for reflux reaction for 3 hours. After the reaction, the excess thionyl chloride was distilled off, and then the fraction at 158°C was collected under the condition of a vacuum of 0.095mPa. The collected fraction was 2-nitro-4-trifluoromethyl Benzoyl chloride;
[0043] b, the preparation of Nitisinone crude product:
[0044] 2-nitro-4-trifluoromethylbenzoyl chloride, 1,3-c...
Embodiment 3
[0048] The preparation method of Nitisinone of the present invention, the detailed steps of this preparation method are as follows:
[0049] The preparation of a, 2-nitro-4-trifluoromethylbenzoyl chloride:
[0050] Taking 2-nitro-4-trifluoromethylbenzoic acid and sulfur oxychloride as raw material, the mol ratio of adding amount between the two of 2-nitro-4-trifluoromethylbenzoic acid and sulfur oxychloride is 1:3, take two kinds of raw materials according to the proportioning ratio between the two, add the raw material 2-nitro-4-trifluoromethylbenzoic acid and thionyl chloride that weighed in the reactor, heat to 79 ℃ for reflux reaction for 3 hours. After the reaction, the excess thionyl chloride was distilled off, and then the fraction at 158°C was collected under the condition of a vacuum of 0.095mPa. The collected fraction was 2-nitro-4-trifluoromethyl Benzoyl chloride;
[0051] b, the preparation of Nitisinone crude product:
[0052] 2-nitro-4-trifluoromethylbenzoyl c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com