Method for decreasing pH (potential of hydrogen) value of vanadium precipitation qualified liquid
A vanadium-precipitating qualified liquid and qualified liquid technology, which is applied in the electrolysis process, electrolytic components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
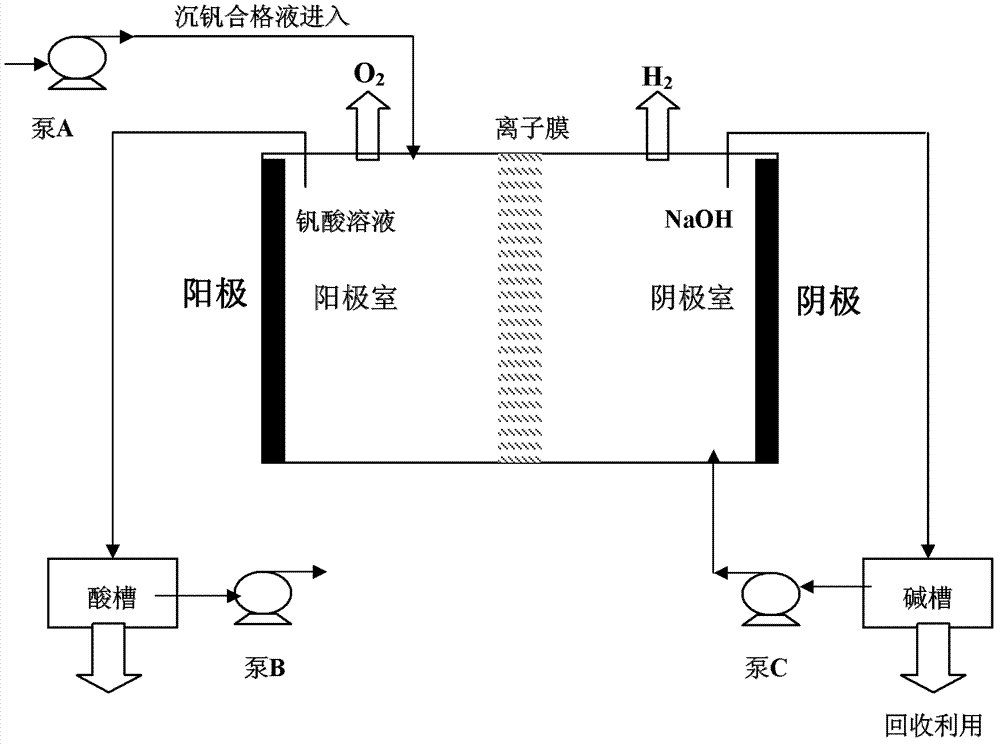
[0030] in such as figure 1The ion-exchange membrane electrolysis device shown performs electrolysis, and the cation-exchange membrane used is a perfluorinated ion-exchange membrane purchased from Asahi Glass Co., Ltd., model number F-8020SP. Among them, 500mL of vanadium-precipitating qualified liquid containing 38.25g / L of vanadium and a pH value greater than 14 is introduced into the anode chamber through pump A, and 500mL of sodium hydroxide solution with a concentration of 0.2% by weight is introduced into the cathode chamber through pump B, and the voltage is controlled. At 5-12V, with a current of 0.05-1.45A, the pH of the solution in the anode compartment reached 2 within 10 hours.
Embodiment 2
[0032] in such as figure 1 The ion-exchange membrane electrolysis device shown performs electrolysis, and the cation-exchange membrane used is the cation-exchange membrane of Zhejiang Qianqiu Environmental Protection Water Treatment Co., Ltd. Among them, 500mL of vanadium-precipitating qualified liquid containing 38.25g / L of vanadium and a pH value greater than 14 is introduced into the anode chamber through pump A, and 500mL of sodium hydroxide solution with a concentration of 0.1% by weight is introduced into the cathode chamber through pump B, and the voltage is controlled. At 5-10V, with a current of 0.05-1.45A, the pH of the solution in the anode compartment reached 2 within 12 hours.
Embodiment 3
[0034] in such as figure 1 The shown ion membrane electrolysis device performs electrolysis, and the cation exchange membrane used is the Nafion117 ion membrane of DuPont Company. Among them, 500mL of vanadium-precipitating qualified liquid containing 38.25g / L of vanadium and a pH value greater than 14 is introduced into the anode chamber through pump A, and 500mL of sodium hydroxide solution with a concentration of 0.5% by weight is introduced into the cathode chamber through pump B, and the voltage is controlled. At 5-15V, with a current of 0.05-1.45A, the pH of the solution in the anode compartment reached 2 within 9 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com