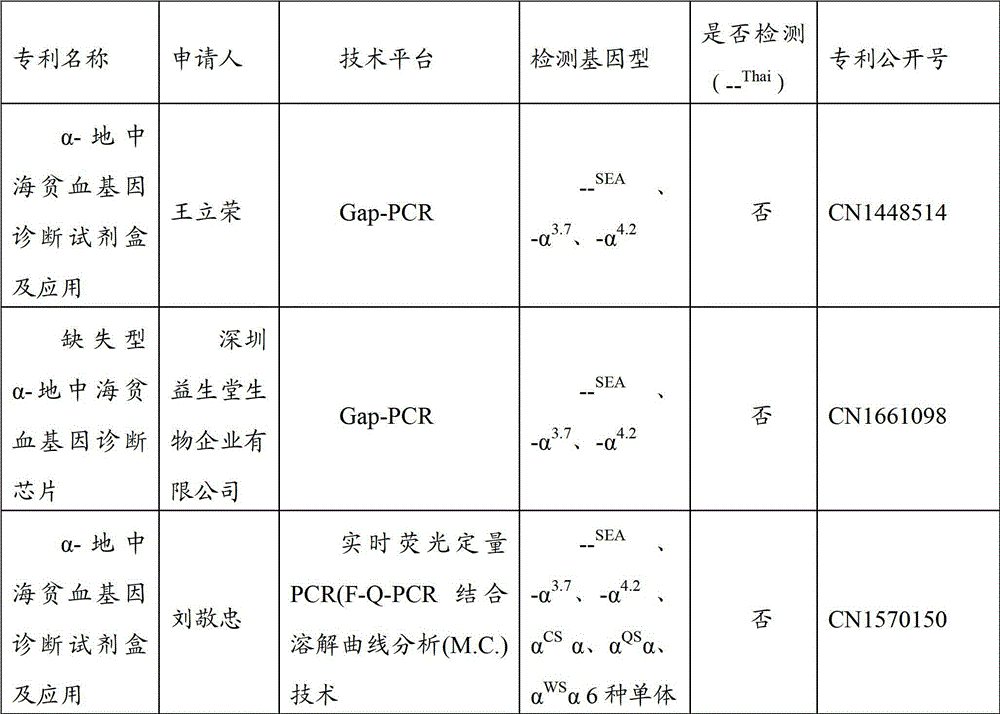
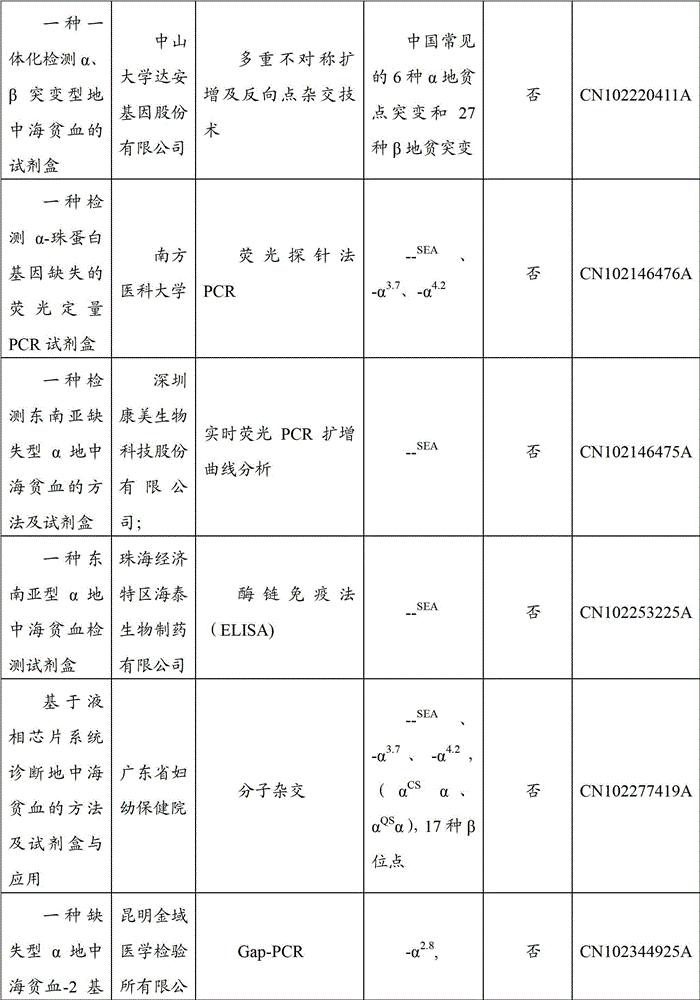
Gene detection kit for Thailand type alpha-thalassemia
A technology for thalassemia and gene detection, which is applied in the field of biomedicine, can solve problems such as time-consuming and laborious, unsuitable for popularization and application, errors and error correction of sequencing platforms, etc., and achieves the effects of convenient use, good social and economic benefits, and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 The composition of the gene detection kit of Thai type α-thalassemia of the present invention
[0040] 1. Primer design and screening:
[0041] Comparative analysis of human α-globin gene sequence, Thai type α-thalassemia (-- Thai ) The upstream of the breakpoint position is 10724-10725 (Genebank No: Z84721), and the downstream is 1219-1220 (Genebank No: Z69706). Primers were designed at the upstream 5' end and downstream 3' end of the breakpoint respectively, THAI-F It binds to the 5' end of the a-globin protein gene cluster, and THAI-R binds to the 3' end of the a-globin protein gene cluster. Utilize orthogonal test and gradient PCR experiment, carry out the screening of primer, screen suitable primer combination, preferred primer combination sequence is as follows:
[0042] THAI-F: AGATCAGTTCCCAGCAAGCCTGGTG;
[0043] THAI-R: GAGATTGCCGATTGTTGAGATTG.
[0044] 2. Optimization of PCR reaction system
[0045] 2 PCR primer storage solution THAI-F and THA...
Embodiment 2
[0061] Embodiment two The detection situation of the kit of the present invention to the clinical sample
[0062] 1. With the results of clinical blood routine and hemoglobin electrophoresis as contrast, 100 samples with abnormal blood routine were detected by using the kit of the present invention. Three cases of Thai type α-thalassemia (-- Thai ) patients, while other genotypes and normal samples were not detected. The detection result of the kit of the present invention is consistent with the results of blood routine and hemoglobin electrophoresis. The screened samples were subjected to gold standard sequencing analysis, and the test results are shown in Table 3: The test results of the kit of the present invention on clinical samples, and the results of statistical analysis and comparison are shown in Table 4: Comparison between the test results of the kit of the present invention and the sequencing results.
[0063] table 3
[0064]
[0065]
[0066]
[0067] ...
Embodiment 3
[0078] Embodiment three The clinical application of kit of the present invention
[0079] 1. Purpose
[0080] For the rare genotype Thai type in α-thalassemia (-- Thai ) to provide a reliable basis for genetic screening of thalassemia. In clinical application, when the patient's clinical manifestations are -- SEA Homozygous, α-thalassemia point mutation (detection of α CS α, α QS α) Not detected, use missing α-thalassemia products on the market (detection-- SEA ,-α 3.7 and-alpha 4.2 ) when doing genetic analysis, only one of the patient's parents carries -- SEA genes, and the other has none-- SEA Gene, can carry Thai type α-thalassemia (-- Thai ) detection. In the second case, the patient has obvious clinical symptoms of standard α-thalassemia, but utilizes the missing α-thalassemia product (test-- SEA ,-α 3.7 and-alpha 4.2 ) detection, missing thalassemia features were not detected, and Thai type α-thalassemia (-- Thai ) detection.
[0081] 2. Inspection princi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com