Carbamate compound and application thereof in antitumor drug
A technology of phenylcarbamate and compound, applied in the field of medicine, can solve the problems of low oral bioavailability, affecting drug absorption and utilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
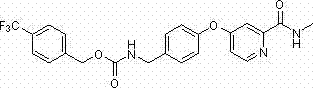
[0088] Example 1: Benzyl-4-(2-(methylcarbamoyl)pyridin-4-oxyl)phenylcarbamate (SH-007)
[0089]
[0090] Weigh 0.710g (0.003 mol) of 4-(4-aminophenoxy)-N-methylpicolinamide into a 100 ml three-neck round bottom flask, add 20 ml of dichloromethane and stir to dissolve, then add 0.4 ml of triethyl amine, under ice bath, slowly add 0.4ml of benzyl chloroformate dropwise, after the dropwise addition, react at room temperature for 1 h, wash with water, extract the organic layer, and separate by column chromatography (eluent: petroleum ether / ethyl acetate = 1: 1), a white solid was obtained. Melting point: 119.2-120.2°C.
[0091] 1 H NMR (CDCl 3 ) δ (ppm): 8.37 ~ 8.35 (d, 1H, pyridine- H ), 8.01 (s, 1H, N H CO), 7.70 (s, 1H, pyridine- H ), 7.47 ~ 7.34 (m, 7H, Ar- H, ), 7.05 ~ 7.02 (d, 2H, Ar- H ), 6.95 ~ 6.92 (m, 1H, pyridine- H ), 6.79 (s, 1H, N H CO), 5.22 (s, 2H, C H 2 ), 3.01 ~ 3.00 (d, 3H, CH 3 ).
[0092] ESI-MS m / z: 378 (M+1) + , calculated value: 378
Embodiment 2
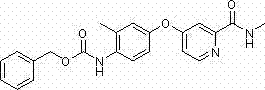
[0093] Example 2: Benzyl-2-methyl-4-(2-(methylcarbamoyl)pyridin-4-oxyl)phenylcarbamate (SH-019)
[0094]
[0095] Using the method of Example 1, 4-(4-aminophenoxy)-N-picolinamide is replaced with 4-(4-amino-3-methylphenoxy)-N-picolinamide, The target compound was obtained as off-white solid. Melting point: 144.4-144.7°C.
[0096] 1 H NMR (CDCl 3 ) δ (ppm): 8.37 ~ 8.35 (d, 1H, pyridine- H ), 8.04 (s, 1H, N H CO), 7.87 ~ 7.84 (s, 1H, N H CO), 7.70 ~ 7.69 (s, 1H, pyridine- H ), 7.44 ~ 7.33 (m, 5H, Ar- H ), 6.96 ~ 6.90 (m, 3H, Ar- H , pyridine- H ), 6.46 (s, 1H, Ar- H ), 5.22 (s, 2H, C H 2 ), 3.01 ~ 3.00 (d, 3H, C H 3 ), 2.25 (s, 3H, C H 3 ).
[0097] ESI-MS m / z: 392 (M+1) + , calculated value: 392.
Embodiment 3
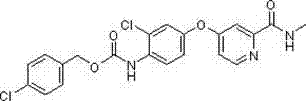
[0098] Example 3: Benzyl-2-chloro-4-(2-(methylcarbamoyl)pyridin-4-oxyl)phenylcarbamate (SH-020)
[0099]
[0100] Using the method of Example 1, 4-(4-amino-3-chlorophenoxy)-N-methylpicolinamide is replaced by 4-(4-aminophenoxy)-N-methylpicolinamide to obtain this The target compound, a brownish-yellow solid. Melting point: 133.4-134.7°C.
[0101] 1 H NMR (CDCl 3 ) δ (ppm): 8.40 ~ 8.38 (d, 1H, pyridine- H ), 8.27 ~ 8.25 (d, 1H, N H CO), 8.00 (s, 1H, N H CO), 7.70 ~ 7.69 (d, 1H, pyridine- H ), 7.45 ~ 7.35 (m, 5H, Ar- H ), 7.17 (s, 1H, Ar- H ), 7.14 ~ 7.13 (d, 1H, Ar- H ), 7.04 ~ 7.02 (m, 1H, Ar- H ), 6.96 ~ 6.94 (m, 1H, pyridine- H ), 5.24 (s, 2H, C H 2 ), 3.01 ~ 3.00 (d, 3H, C H 3 ).
[0102] ESI-MS m / z: 412 (M+1) + , calculated value: 412.
[0103]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com