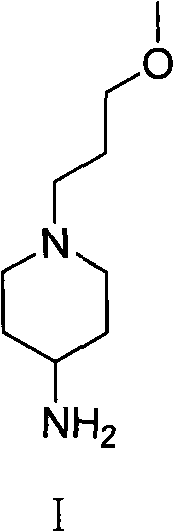
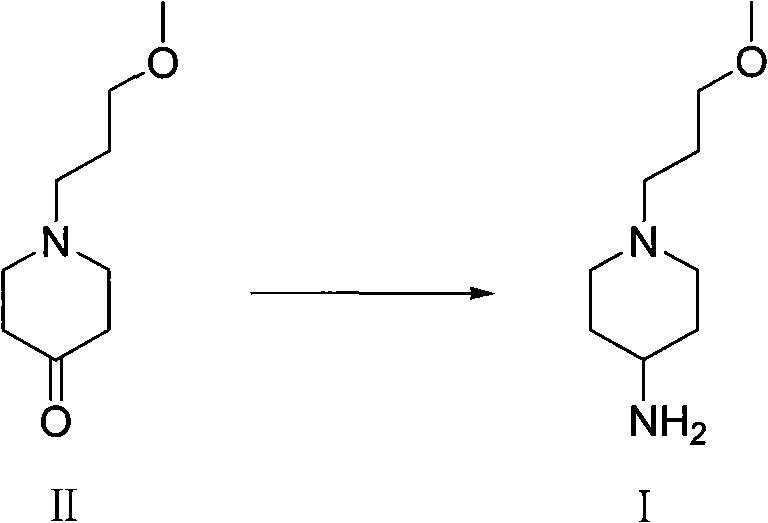
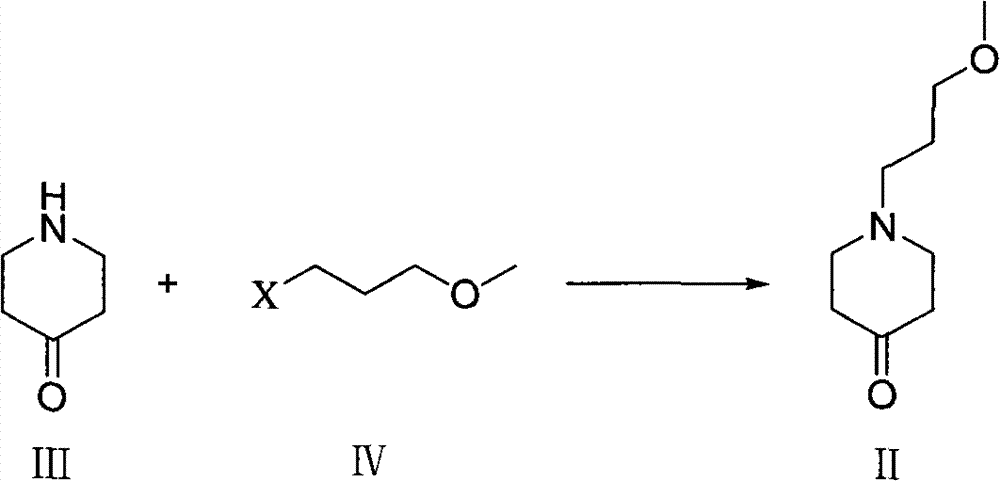
Method for preparing 1-( 3-methoxy propyl )- 4-piperidine amine and salt thereof
A technology of methoxypropyl and piperidinamine, which is applied in the field of preparation of 1--4-piperidinamine and its salts, can solve the problems of unseen synthesis, achieve cheap raw materials, easy access to raw materials, and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthesis of embodiment 1 prucalopride
[0023] Add 4-amino-5-chloro-dihydro-7-benzofuran carboxylic acid (1.0g, 4.68mmol) and 10ml tetrahydrofuran into a 50ml three-necked flask, stir for 15 minutes and add carbonyldiimidazole (0.98g, 6.08mmol) , under nitrogen protection, stirred at room temperature for 30 minutes. A solution of 1-(3-methoxypropyl)-4-piperidinamine (1.04 g, 6.08 mmol) in 10 ml of tetrahydrofuran was added dropwise, and the temperature was raised to 50° C. after the drop, and reacted for 5 hours. Cool, evaporate the solvent to dryness under reduced pressure, add 20ml of water to the residue, stir for 2 hours, filter, and dry the filter cake to obtain 1.4g of off-white solid, yield 81.5%, mp.98-102°C, and its structural identification data are as follows:
[0024] MS(m / z): 368[M+H] + ;
[0025] 1 HNMR (CDCl 3 )δ1.95~2.02(m, 4H), 2.17~2.20(d, 2H), 2.72(t, 2H), 2.99(t, 2H), 3.07(t, 2H), 3.33(s, 3H), 3.45 (t, 4H), 4.15-4.19 (m, 1H), 4.27 (s, 2H),...
Embodiment 2
[0026] Example 2 Synthesis of 1-(3-methoxypropyl)-4-piperidone
[0027] Add 4-piperidone hydrochloride monohydrate (10g, 0.065mol), potassium carbonate (11.04g, 0.08mol), 3-bromopropyl methyl ether (12.85g, 0.084mol) successively in a 250ml single-necked bottle ) and 100ml of acetonitrile were reacted at 50°C for 17 hours. Cool, filter with suction, remove potassium carbonate, evaporate the filtrate to dryness under reduced pressure, and obtain 13 g of a dark red oily substance, which is directly used in the next reaction.
Embodiment 3
[0028] Example 3 Synthesis of 1-(3-methoxypropyl)-4-piperidinamine and its hydrochloride
[0029] In the high-pressure hydrogenation kettle, add the obtained 1-(3-methoxypropyl)-4-piperidone (5g, 0.029mol), saturated methanol solution (60ml) of ammonia gas and 10% Pd / C (0.5g), hydrogenated to 1.5MPa, stirred at 40°C for 7 hours. Cool, filter with suction, evaporate the filtrate to dryness under reduced pressure to obtain a yellow oil, dissolve it in 20ml of ethanol, add 5mol / L ethyl hydrogen chloride solution dropwise, adjust the pH to 1-2, stir for 2 hours, filter, The filter cake was washed with an appropriate amount of ethanol to obtain 4.34 g of white solid 1-(3-methoxypropyl)-4-piperidinamine hydrochloride, mp>220°C, and the total yield of the two-step reaction was 70.8%.
[0030] Dissolve 1-(3-methoxypropyl)-4-piperidinamine hydrochloride (4g, 0.016mol) in 40ml of methanol, add potassium carbonate (4.5g, 0.032mol), stir at room temperature for 2 hours, pump After filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com