Method for synthesizing 4-dihydroxyborane-2-fluorophenylalanine
A technology of fluorophenylalanine and dihydroxyborane, applied in the field of synthesis of 4-dihydroxyborane-2-fluorophenylalanine, achieving high yield, easy purification, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
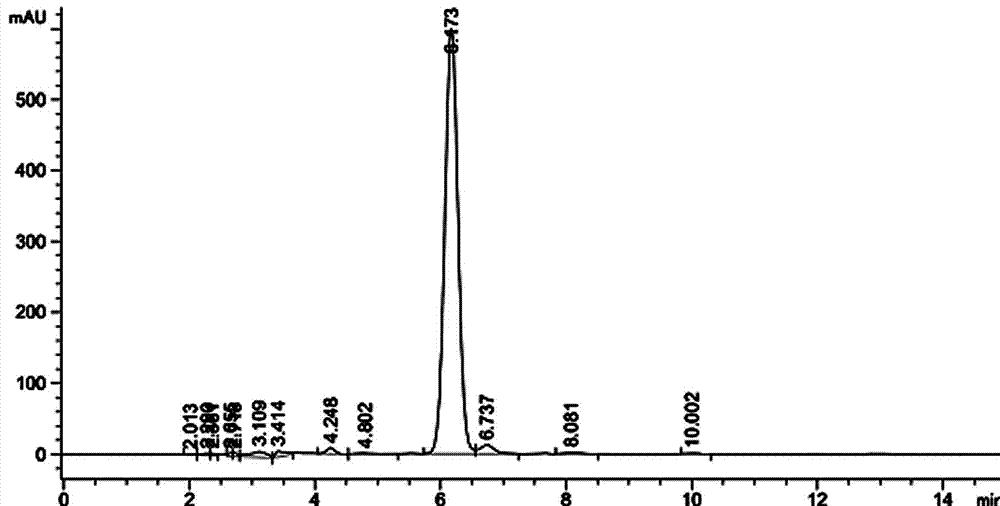
Image
Examples
Embodiment Construction
[0027] The present invention is described in further detail below in conjunction with embodiment.
[0028] Nitration reaction: Dissolve 6.63g of p-iodotoluene (1) in 5mL of acetic anhydride, control the temperature at 0°C, stir magnetically, slowly add concentrated 3mL of HNO 3 After the addition, the temperature was controlled to 20-25°C, reacted for 4 hours, cooled to room temperature, and added NaOH solution to adjust the pH to 7, the mixture was extracted 3 times with 200 mL of ethyl acetate, washed with saturated brine, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, mix the sample with silica gel, petroleum ether: ethyl acetate (v:v) = 1:3-1:5 as the mobile phase to pass through the column, and rotary evaporate at 25°C to obtain 2.80 g of p-iodo-o-nitrotoluene (2) as a yellow oil , the yield is 35%.
[0029] Bromination reaction: Dissolve 2.63 g of p-iodo-o-nitrotoluene (2) in 50 mL of carbon tetrachloride, then add 1.78 g of N-bromosuccinimide (NBS) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com