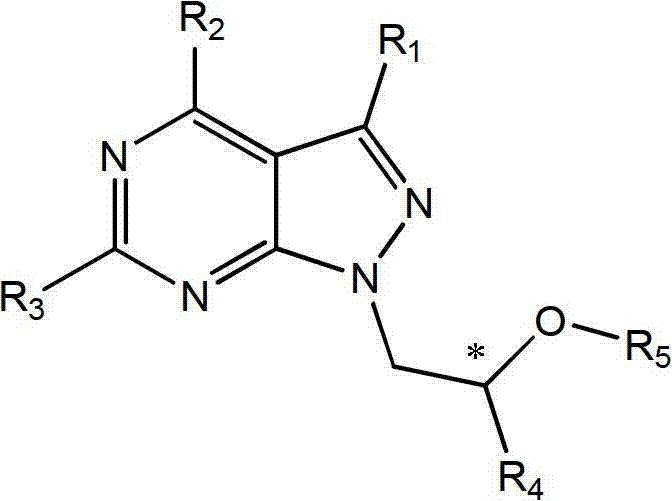
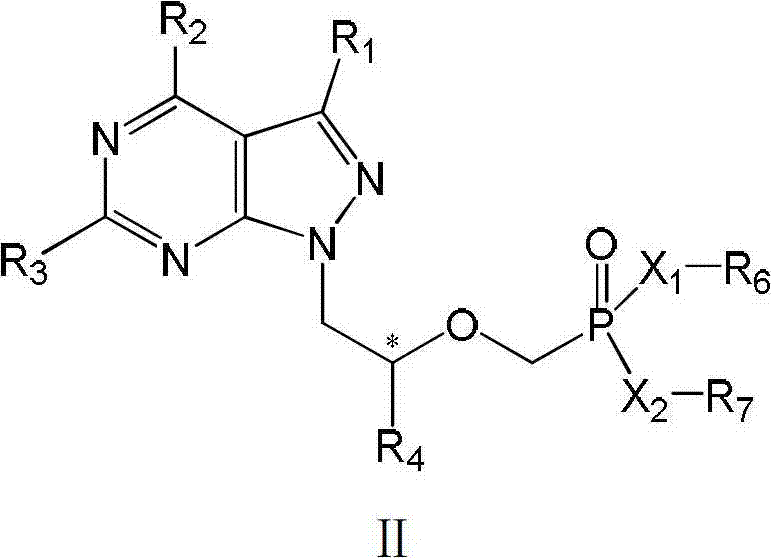
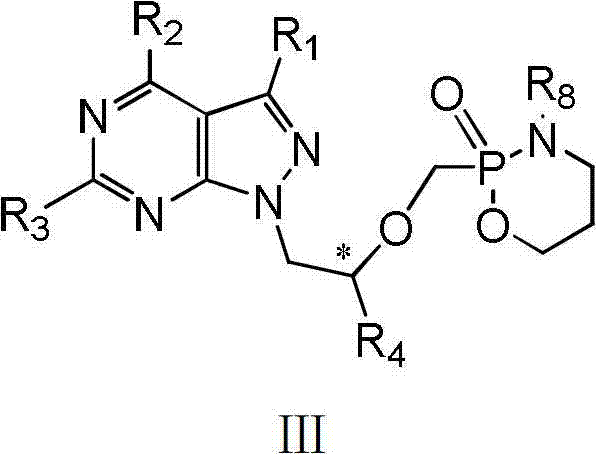
Acyclic nucleotide analogs as well as preparation method and application thereof
A technology of acyclic nucleotides and analogues, which is applied in the field of chemical synthesis of drugs and can solve problems such as drug resistance and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] The preparation of embodiment 1 (R)-1-(2-hydroxypropyl)-4-aminopyrazolo[3,4-d]pyrimidine
[0090]
[0091] Add 5.26g of 4-aminopyrazolo[3,4-d]pyrimidine to a 250mL round bottom flask, then add 200mL of DMF and 83mg of sodium hydroxide, heat the reaction solution to 140°C, slowly add 3.90mL of R-carbonic acid Acrylate, react at 140°C for 16 hours after addition. TLC (DCM:MeOH=8:1) detected that the reaction was complete. The solvent was distilled off under reduced pressure and separated by silica gel column chromatography to obtain 5.55 g of a white solid with a yield of 74.7%.
[0092] 1 H NMR (DMSO-d 6 ,400Hz)δ8.16(s,1H),8.08(s,1H),7.63(s,2H),4.28-4.22(m,1H),4.13(d,2H,J=5.6Hz),3.37(s ,1H), 1.03(d,3H,J=5.6Hz).
Embodiment 2
[0093] Example 2 (R)-N 4 - Preparation of dimethylaminomethylene-1-(2-hydroxypropyl)-pyrazolo[3,4-d]pyrimidine
[0094]
[0095] In a 100mL flask, add 11.24g (R)-1-(2-hydroxypropyl)-4-aminopyrazolo[3,4-d]pyrimidine, 23.5mLN,N-dimethylformamide dimethyl acetal (DMF-DMA), and then add 50 mL of chloroform. Under nitrogen protection, stir overnight at room temperature. TLC detection (DCM:MeOH=9:1) until the reaction is complete. The solvent was distilled off under reduced pressure to obtain a pale yellow oil, which was separated by silica gel column chromatography to obtain 11.4 g of a white solid with a yield of 78.9%.
Embodiment 3
[0096] Example 3 (R)-N 4 - Preparation of dimethylaminomethylene-1-[2-bis(ethylphosphonomethoxy)propyl]-pyrazolo[3,4-d]pyrimidine
[0097]
[0098] In a 500mL flask, add 11.4g (R)-N 4 -Dimethylaminomethylene-1-(2-hydroxypropyl)-pyrazolo[3,4-d]pyrimidine and 14.1 mL of diethyl p-toluenesulfonyloxymethylphosphonate, then 300 mL of toluene was added, Add 5.51gNaH under ice-salt bath. Under nitrogen protection, stir overnight at room temperature. TLC detection (DCM:MeOH=10:1) until the reaction was complete. The solvent was distilled off under reduced pressure, the solid was dissolved in 100ml of dichloromethane, and glacial acetic acid was added drop by drop under stirring to adjust the pH to 8-9. It was extracted three times with dichloromethane, dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain a yellow oil. Separated by silica gel column chromatography, 11.01 g of a white solid was obtained with a yield of 60.2%. 1 HNMR (CDCl 3 ,400...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com