Method for detecting antibacterial drugs of furazolidone and furacilin through surface-enhanced raman spectroscopy
A furazolidone nitrofuracil and surface-enhanced Raman technology, which can be used in the fields of feed, food, and drug testing, and can solve the problems of low sensitivity, direct abuse, and enhanced bacterial resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
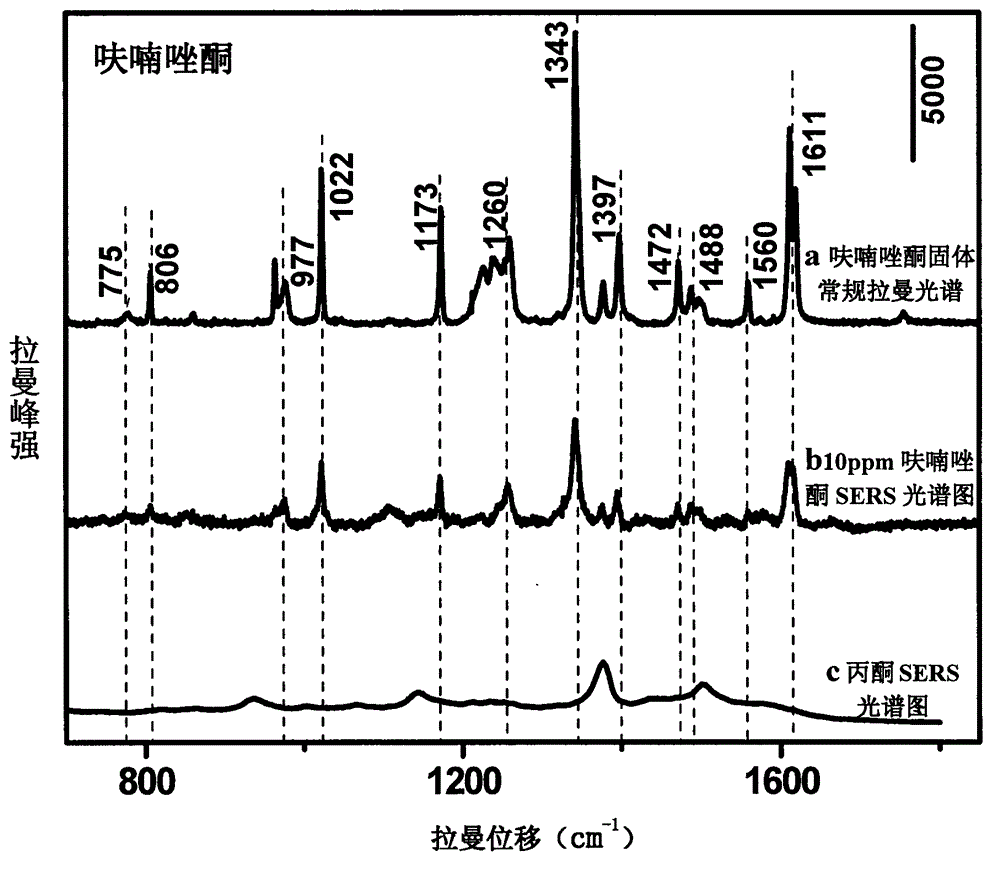
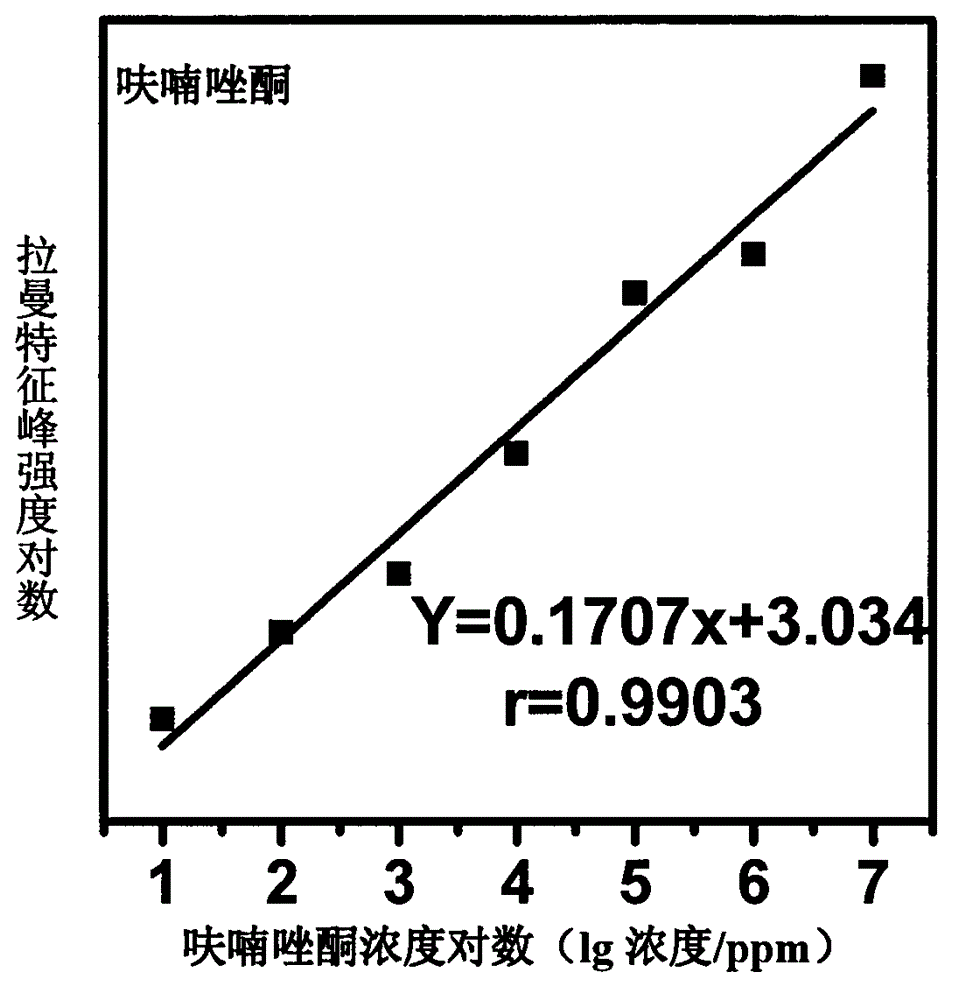
[0018] Use acetone to prepare furazolidone into solutions with concentrations of 1000, 750, 100, 50, 10, 5, and 1 ppm, mix them with gold colloid at a ratio of 1:10, and use 1M HNO 3 solution or 1M NaOH solution to adjust the pH to 1.0-6.0, and scan the Raman spectrum to obtain the surface-enhanced Raman spectrum of each concentration gradient solution; mix acetone and gold sol for Raman detection to obtain the background Raman spectrum ; The surface-enhanced Raman spectrum of furazolidone solution is compared with the conventional Raman spectrum of furazolidone standard after deducting the background Raman spectrum, and it is determined that the characteristic peak is 1602cm -1 , 1554cm -1 , 1456cm -1 , 1326cm -1 , 1242cm -1 , 1162cm -1 , 968cm -1 It is used for qualitative; and the concentration is used as the abscissa, and the characteristic peak is 1008cm -1 The peak height is the ordinate, and a standard curve is made for quantification; the linear equation of the s...
Embodiment 2
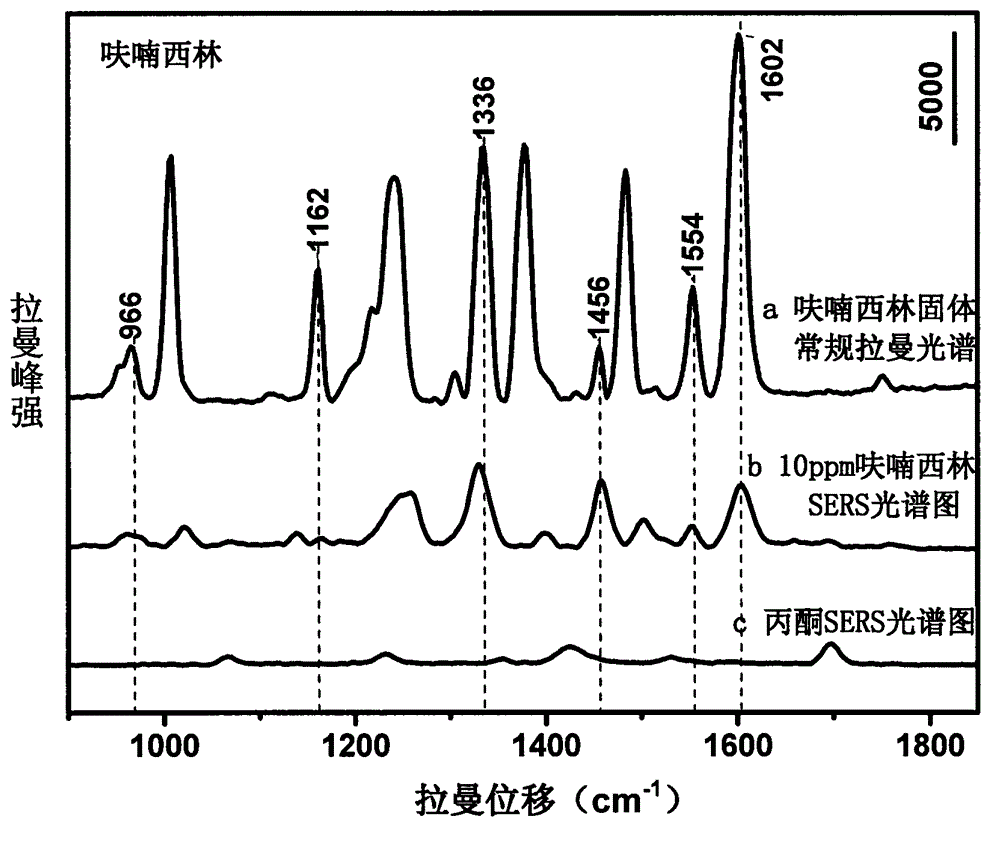
[0020] Use acetone to prepare nitrofurazone into solutions with concentrations of 1000, 750, 100, 50, 10, 5, and 1 ppm, mix them with gold colloid at a ratio of 1:10, and use 1M HNO 3 solution or 1M NaOH solution to adjust the pH to 1.0-6.0, and scan the Raman spectrum to obtain the surface-enhanced Raman spectrum of each concentration gradient solution; mix acetone and gold sol for Raman detection to obtain the background Raman spectrum ; The surface-enhanced Raman spectrum of the nitrofurazone solution is compared with the conventional Raman spectrum of the furazolidone standard after deducting the background Raman spectrum, and the characteristic peak is determined to be 1602cm -1 , 1556cm -1 , 1484cm -1 , 1388cm -1 , 1336cm -1 , 1008cm -1 , 968cm -1 It is used for qualitative; and the concentration is used as the abscissa, and the characteristic peak is 1162cm -1 Peak height is ordinate, and standard curve is made in order to quantify; The linear equation that obtain...
Embodiment 3
[0022] Accurately weigh 2g of compound feed sample, add 50ml of acetone, ultrasonically extract in a 65°C water bath for 15min, and centrifuge at 3500r / min for 10min. Take 5ml of the supernatant and concentrate it to near dryness by rotary evaporation, and dilute to 2ml with acetone for Raman detection of furazolidone. A total of three parallel samples were made, and the analysis results are shown in Table 1; it can be seen from the analysis that the relative standard deviation of the parallel samples is 2.453%.
[0023] Table 1 The detection results of furazolidone in compound feed samples
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com