Drug for inflammatory bowel disease
A technology for inflammatory bowel disease, medicine, applied in the field of medicine for inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
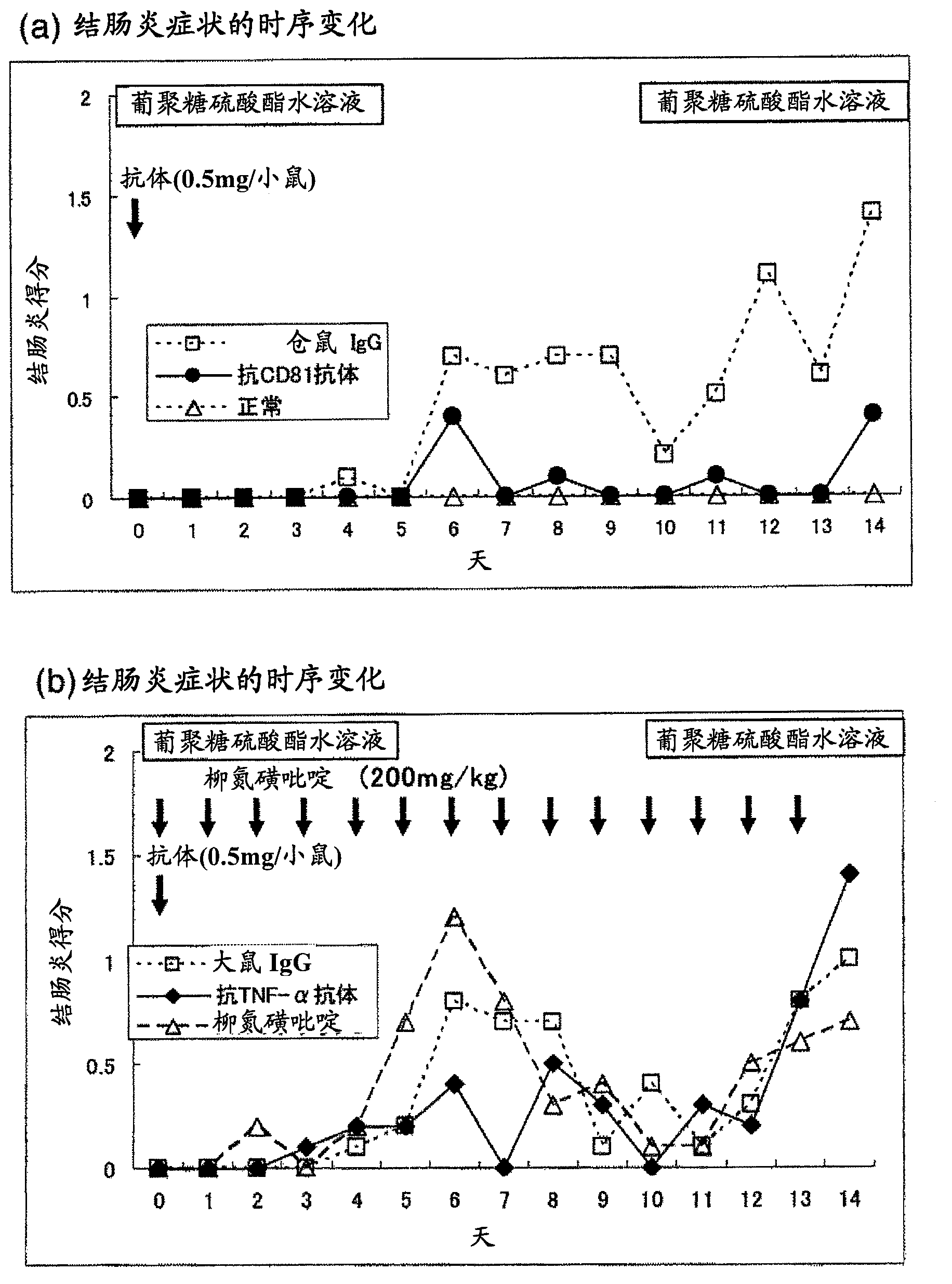
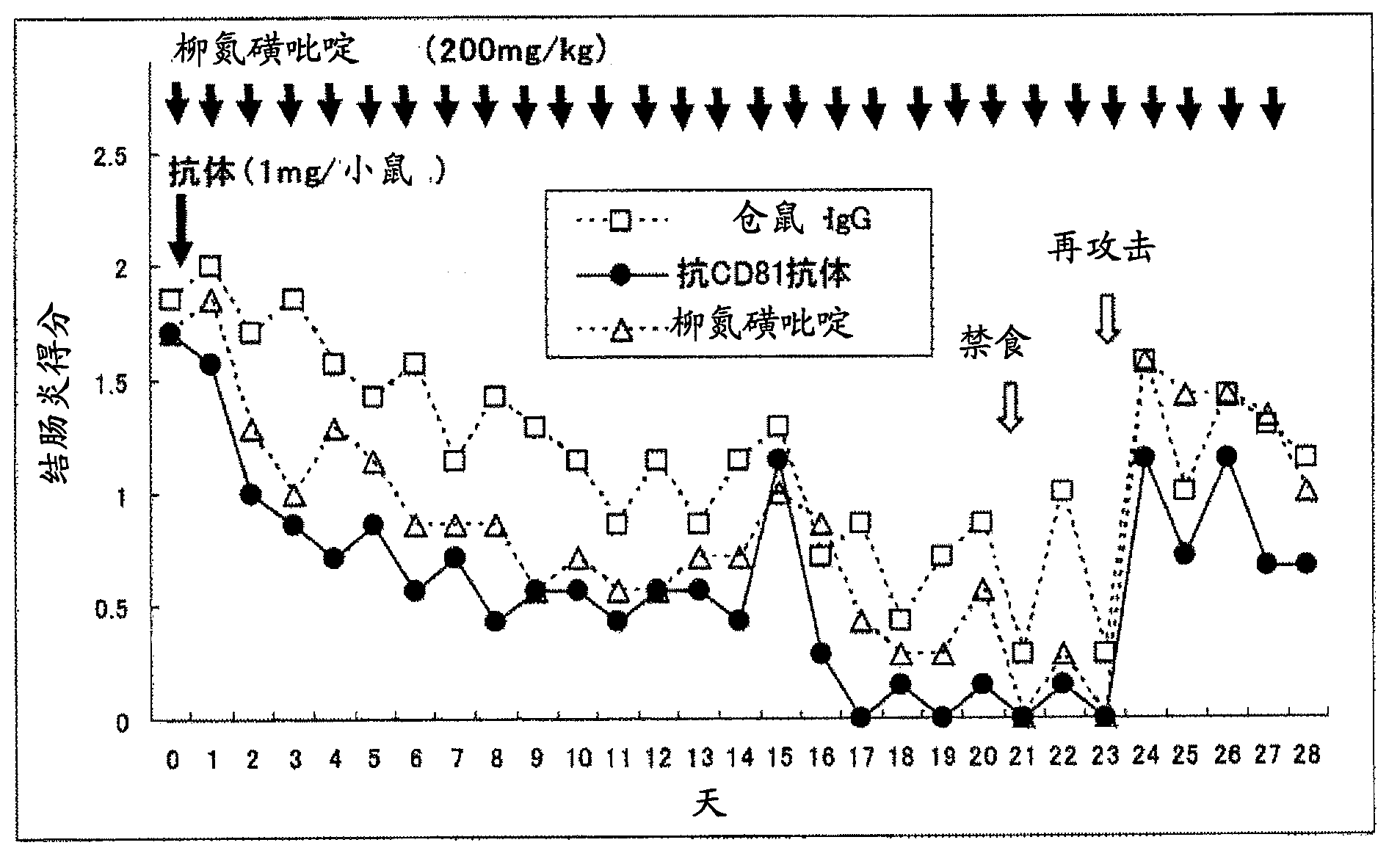
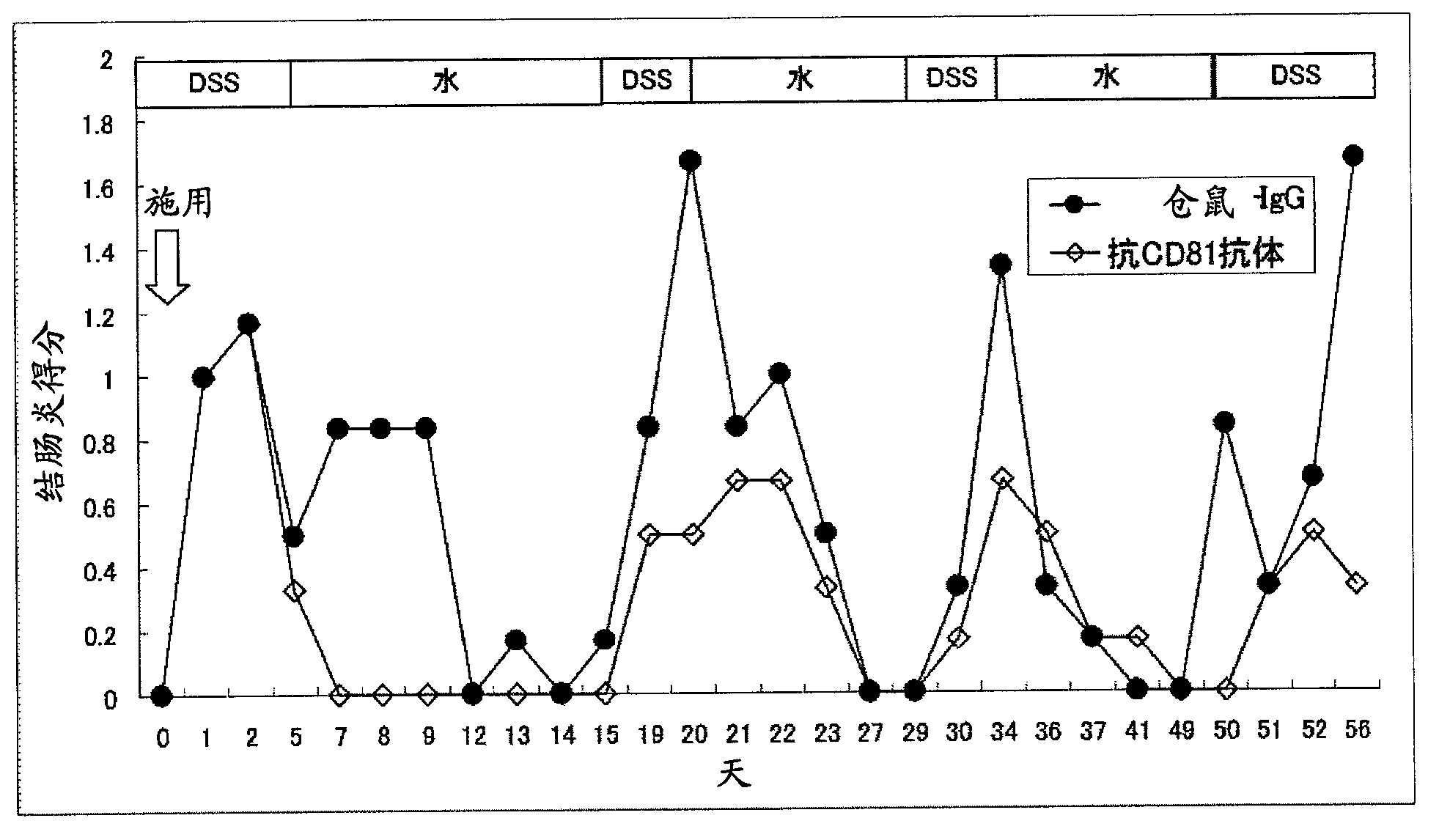
[0125] Single dose in mouse model of colitis induced with dextran sulfate (DSS) aqueous solution Relapse suppression effect of administration of anti-CD81 antibody
[0126] An aqueous solution of dextran sulfate (DSS) was administered to mice to induce relapsing-remitting ulcerative colitis. Anti-CD81 antibody was administered once to this relapsing-remitting ulcerative colitis mouse model, and it was combined with a single dose of anti-TNF-α antibody and multiple doses of sulfasalazine (both existing treatments). The inhibitory effect of anti-CD81 antibody administered in a single dose on the relapse (reoccurrence) of ulcerative colitis was examined in comparison with a single dose.
[0127] 1. Method
[0128] (1) Preparation of DSS
[0129] Dextran sulfate (product of TdB consultancy AB, average molecular weight 47,000) was dissolved in drinking water to prepare a 1% aqueous solution.
[0130] (2) Preparation of application solution
[0131] For the test drugs ...
Embodiment 2
[0155] Anti-CD81 antibody in mouse model of colitis induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) long-term remission in
[0156] The anti-CD81 antibody was once administered to a mouse model of relapsing-remitting inflammatory bowel disease (crohn's disease and ulcerative colitis) by administering TNBS to Mice were obtained, and the relapse (recurrence)-inhibitory effect of single-dose administration of anti-CD81 antibody on inflammatory bowel disease was examined by comparison with multiple-dose administration of sulfasalazine, an existing therapeutic drug.
[0157] 1 method
[0158] (1) Preparation of TNP-OVA
[0159] Mix 0.5g ovalbumin (OVA: Sigma) and 0.5g K 2 CO 3 (Nacalai Tesque, Inc.) was dissolved in 25 ml of distilled water for injection (OVA solution). Dissolve 0.5 g of 2,4,6-trinitrobenzenesulfonic acid (TNBS: Nacalai Tesque, Inc.) in 25 ml of 0.1 M K 2 CO 3 Medium (TNBS solution). The OVA solution and TNBS solution were mixed and stirred over...
Embodiment 3
[0173] In the colitis mouse model induced by dextran sulfate (DSS) aqueous solution, a single dose of Relapse Inhibitory Effect of Anti-CD81 Antibody
[0174] Anti-CD81 antibody was administered once to a mouse model of relapsing-remitting ulcerative colitis obtained by administering dextran sulfate (DSS) aqueous solution to mice, and the effect of anti-CD81 antibody on longer-term induction was examined. Relapse inhibition in relapsing-remitting ulcerative colitis.
[0175] 1. Method
[0176] (1) Preparation of DSS
[0177] Dextran sulfate (product of Wako Pure Chemical Industries, Ltd., average molecular weight 5,000) was dissolved in drinking water to prepare a 2% aqueous solution.
[0178] (2) Preparation of application solution
[0179] For the test drug under study, hamster anti-CD81 antibody was used, and for the pathological condition control test drug, hamster IgG was used. A concentrated stock solution of hamster anti-CD81 antibody (clone: 2F7, product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com