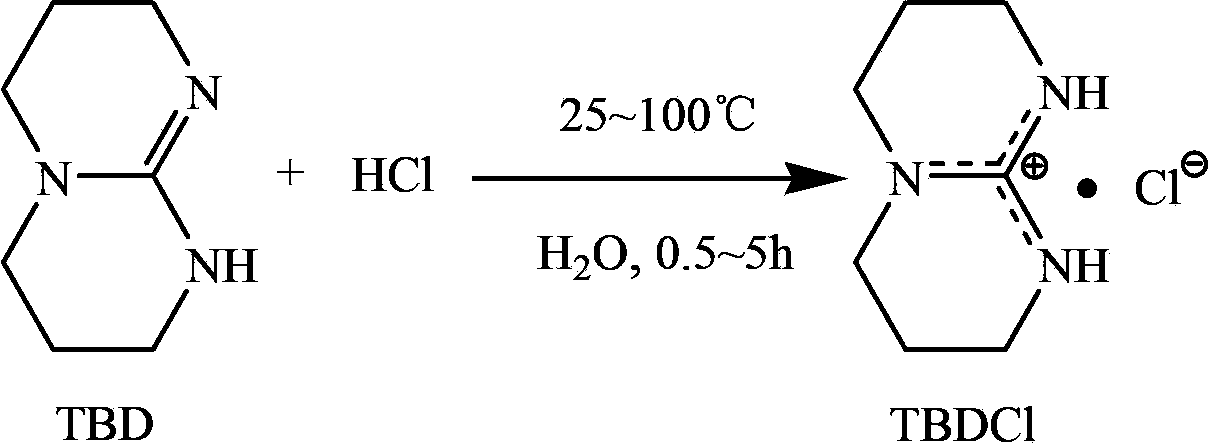
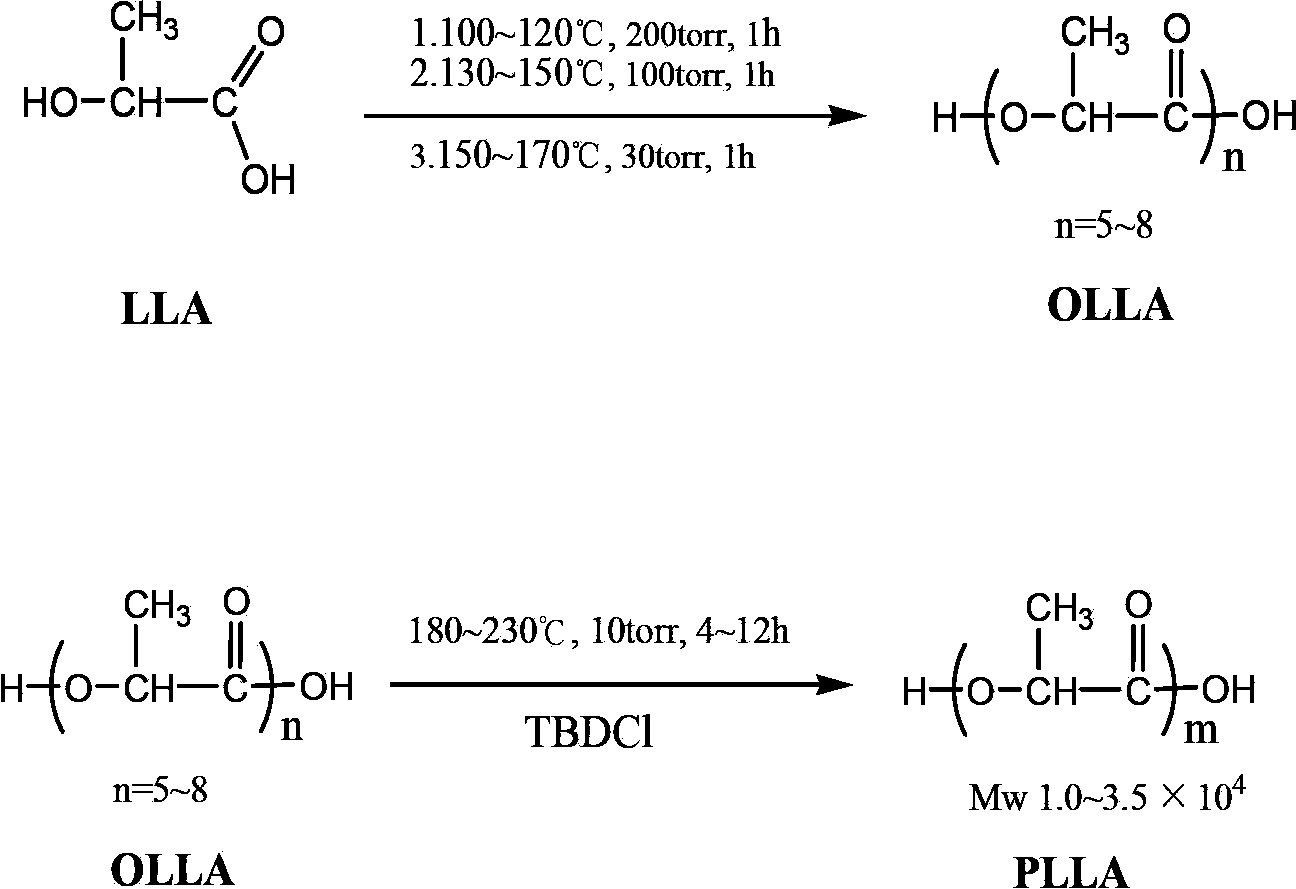
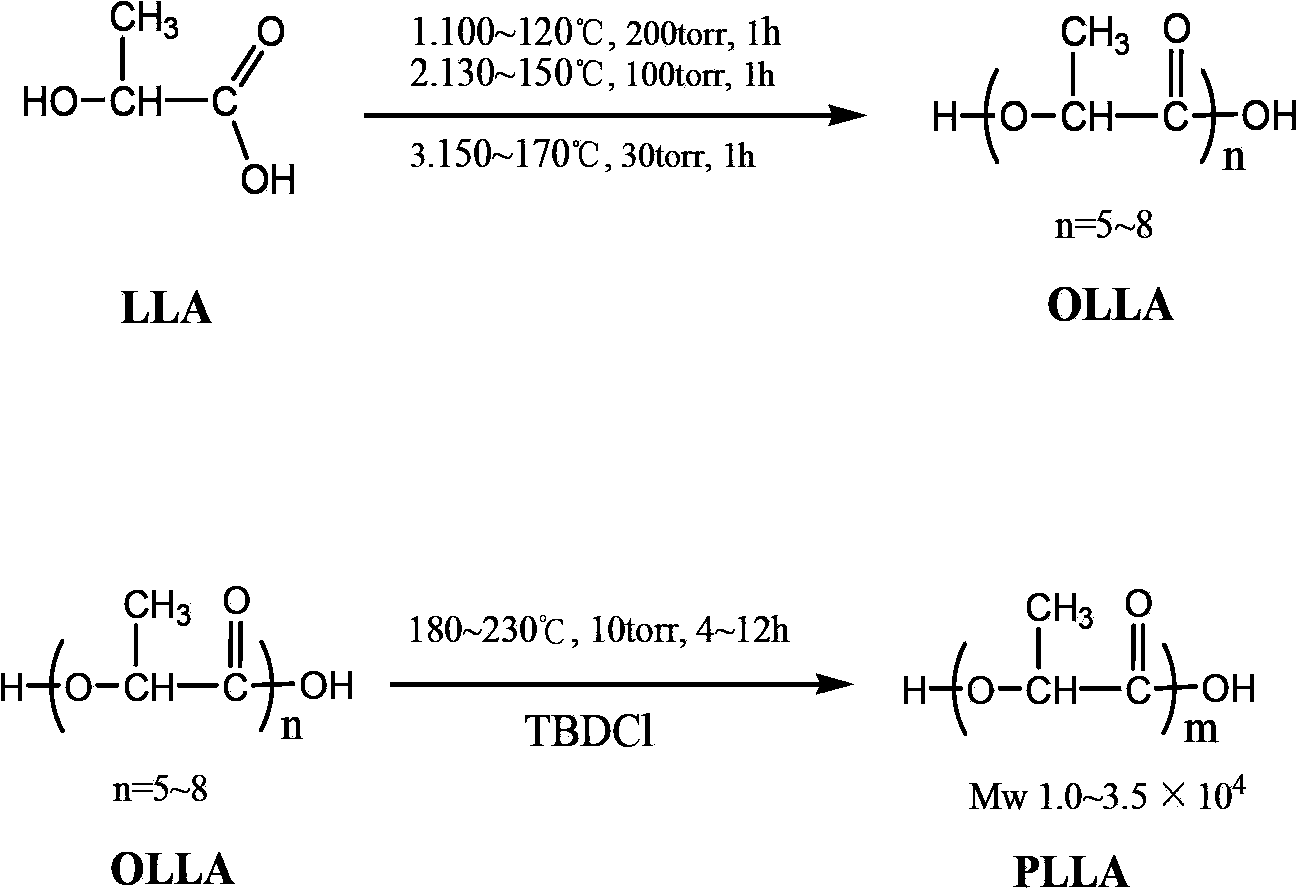
Technical method for synthesizing medical biodegradable polylactic acid by catalyzing condensation polymerization of lactic acid through chlorinated bicyclic guanidine
A technology of bicyclic guanidine catalyzing lactic acid and biodegradability is applied in the field of drug degradation materials, which can solve the problems of long catalytic reaction time and hidden danger of product biosafety, and achieve the effects of easy industrialization, low cost of raw materials, and simple reaction process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 20mL of deionized water into the Schleck reaction kettle, then add 2g (14.4mmol) of bicyclic guanidine, heat and stir under the protection of high-purity argon, after the rune reaches the pre-selected temperature of 25°C, add the concentration to the kettle dropwise from the constant pressure dropping funnel 2% hydrochloric acid aqueous solution, control the molar ratio of bicyclic guanidine and hydrochloric acid to 1:1.1, and stir for 5h. The obtained product was rotary evaporated to remove the contained water, and the solid product was moved into a vacuum drying oven to dry for 48 hours to obtain 2.41 g of a white solid product, namely bicyclic guanidine chloride, with a yield of 95.6%.
Embodiment 2
[0027] Add 30mL of deionized water into the Schleck reactor, then add 2g (14.4mmol) of bicyclic guanidine, heat and stir under the protection of high-purity argon, and after the rune reaches the preselected temperature of 55°C, add the concentration to the kettle dropwise from the constant pressure dropping funnel. 4% hydrochloric acid aqueous solution, control the molar ratio of bicyclic guanidine and hydrochloric acid to 1:1.2, and stir for 3 hours. The obtained product was rotary evaporated to remove the contained water, and the solid product was moved into a vacuum drying oven to dry for 48 hours to obtain 2.47 g of a white solid product, namely bicyclic guanidine chloride, with a yield of 98.0%.
Embodiment 3
[0029] Add 20mL of deionized water into the Schleck reaction kettle, then add 2g (14.4mmol) of bicyclic guanidine, heat and stir under the protection of high-purity argon, after the rune reaches the pre-selected temperature of 100°C, add the concentration to the kettle dropwise from the constant pressure dropping funnel 2% hydrochloric acid aqueous solution, control the molar ratio of bicyclic guanidine and hydrochloric acid to 1:1, and stir for 0.5h. The obtained product was rotary evaporated to remove the contained moisture, and the solid product was moved into a vacuum drying oven to dry for 48 hours to obtain 2.34 g of a white solid product, namely bicyclic guanidine chloride, with a yield of 93.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com