Universal hapten of fluoroquinolone medicines, artificial antigen, broad-spectrum monoclonal antibody, preparation method and application
A fluoroquinolone and monoclonal antibody technology, applied in the field of food safety, can solve the problem that antibodies cannot recognize fluoroquinolone drugs, etc., and achieve the effect of satisfying on-site rapid detection, reducing detection cost, and improving detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Experiment 1: Synthesis of a universal hapten for fluoroquinolones
[0038] (a) In a 50ml three-neck flask, add 8ml of distilled water, heat to 80°C, add 1mmol of sarafloxacin standard substance into the bottle, and add dropwise sodium hydroxide solution until dissolved, slowly add 1mmol of 2- Chloroethylamine, stirred slowly for 2 hours, during which sodium hydroxide solution was continued dropwise to maintain pH=8.0;
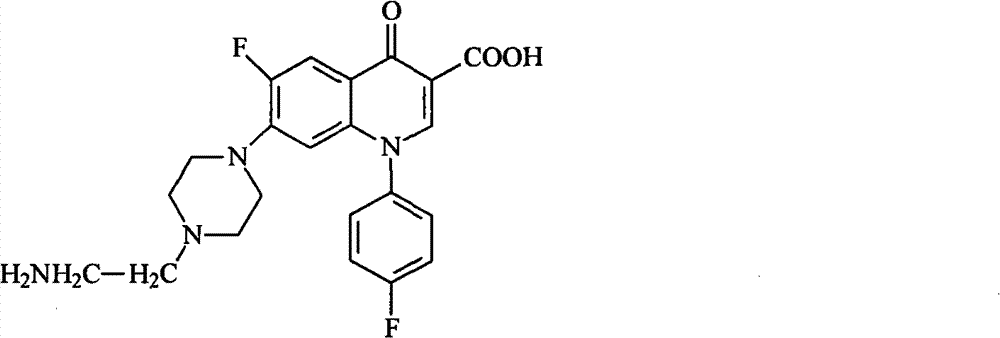
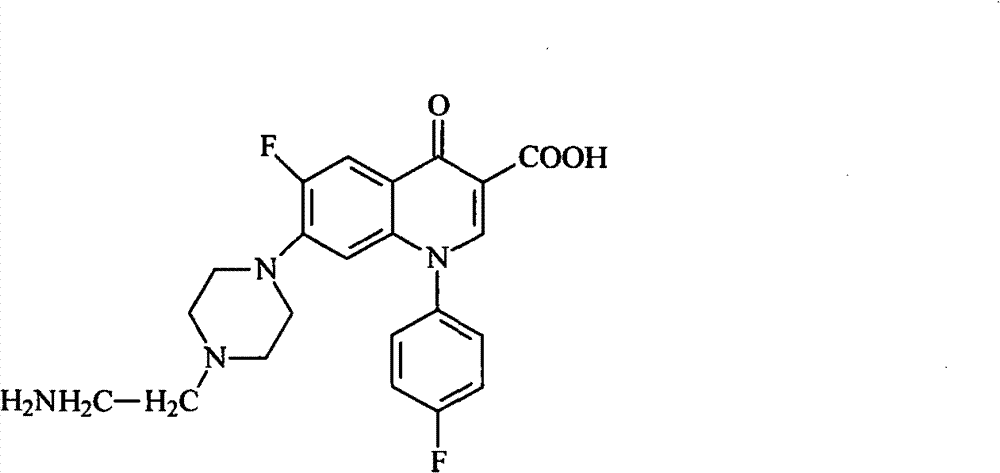
[0039] (b) Lower the temperature of the solution in step (a) to 60°C, add hydrochloric acid to neutralize to pH=6.0, and precipitate a solid, suction filter the obtained solid, and wash with water to obtain the sarafloxacin hapten. The molecular structural formula of product is as shown in formula 1:
[0040]
[0041] Formula 1.
[0042] The product has a melting point of 225°C; infrared spectrum (IR) characterization data: (KBr)V max 3600, 3392, 3075, 2975, 2825, 1733, 1627, 1490, 1259, 1157, 806, 730, 540cm -1 . Compared with the infrared data...
Embodiment 2
[0051] Experiment 1: Synthesis of a universal hapten for fluoroquinolones
[0052] (a) In a 50ml three-neck flask, add 8ml of distilled water, heat to 75°C, add 1mmol of sarafloxacin standard substance into the bottle, and add dropwise sodium hydroxide solution until dissolved, slowly add 1.5mmol of 2 - Chloroethylamine, stirred slowly for 2.5 hours, during which sodium hydroxide solution was continued dropwise to maintain pH=7.5;
[0053] (b) Lower the temperature of the solution in step (a) to 55°C, add hydrochloric acid to neutralize to pH=5.5, and precipitate a solid, suction filter the resulting solid, and wash with water to obtain the sarafloxacin hapten. The molecular structural formula of product is as shown in formula 1:
[0054]
[0055] Formula 1.
[0056] The product structure and characterization data are the same as above.
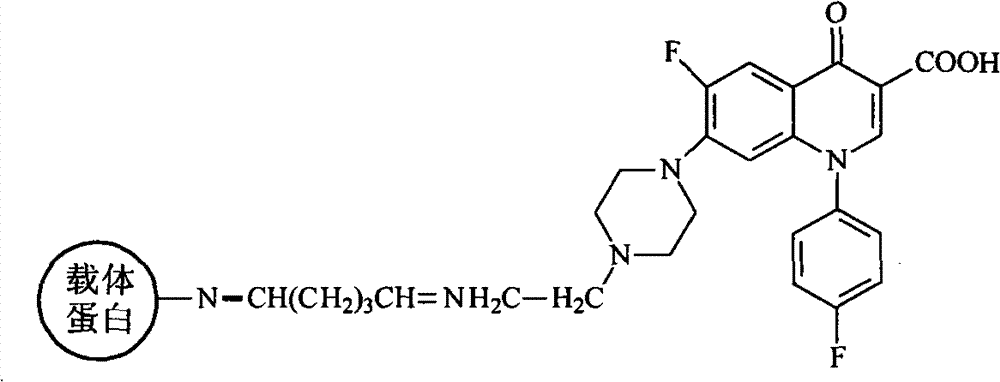
[0057] Experiment 2: Preparation of common artificial antigens for fluoroquinolones
[0058] (a) 60 mmol of sarafloxacin hapten was p...
Embodiment 3
[0065] Experiment 1: Synthesis of a universal hapten for fluoroquinolones
[0066] (a) In a 50ml three-neck flask, add 8ml of distilled water, heat to 85°C, add 1mmol of sarafloxacin standard substance into the bottle, and add dropwise sodium hydroxide solution until dissolved, slowly add 2mmol of 2- Chloroethylamine, stirred slowly for 3 hours, during which sodium hydroxide solution was continued dropwise to maintain pH=8.5;
[0067] (b) Lower the temperature of the solution in step (a) to 65°C, add hydrochloric acid to neutralize to pH=6.5, and precipitate a solid, suction filter the resulting solid, and wash with water to obtain the sarafloxacin hapten. The molecular structural formula of product is as shown in formula 1:
[0068]
[0069] Formula 1.
[0070] The product structure and characterization data are the same as above.
[0071] Experiment 2: Preparation of common artificial antigens for fluoroquinolones
[0072] (a) 50 mmol of sarafloxacin hapten was placed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com