Gene drug co-carrier system using cation liposome to establish gold nano-spherical shell and preparation method
A gold nanosphere shell and cation technology, applied in gene therapy, drug combination, pharmaceutical formulation, etc., can solve the problems of difficult removal of impurities, gene instability, gene decomposition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
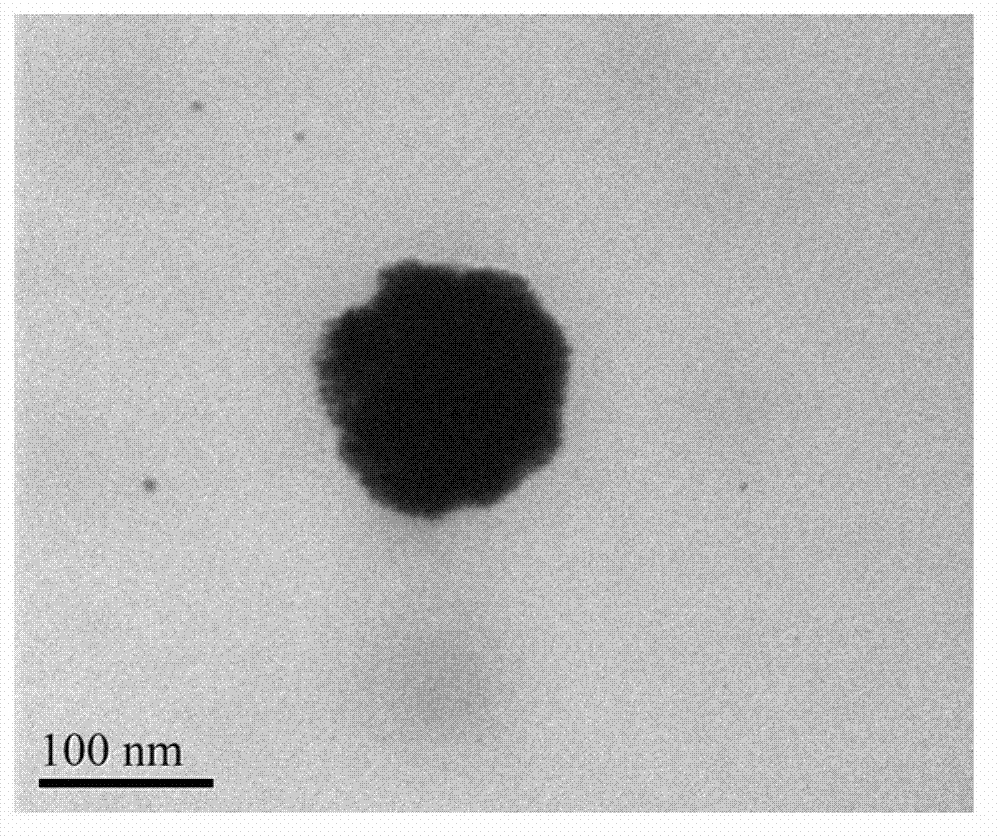
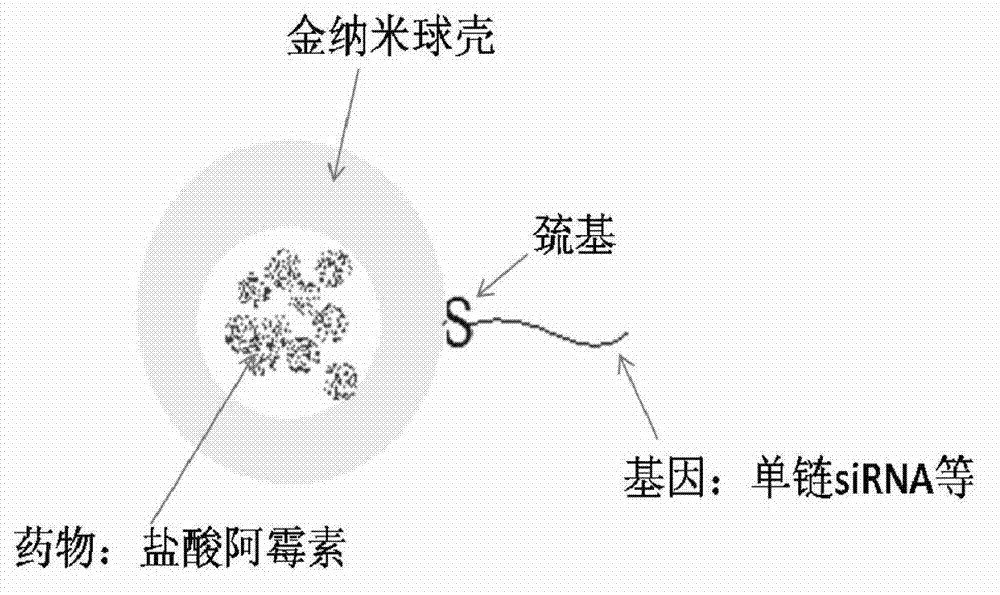
[0022] a. Preparation of cationic liposomes: DPPC, MPPC, and DPPE-PEG2000 were dissolved in PBS solution (pH=7.4) according to the weight ratio of 40:40:10 to form a 60nM lipid solution, and doxorubicin hydrochloride was added, The concentration of doxorubicin hydrochloride was 5nM, and the solution was prepared into liposomes according to the standard cycle freeze-thaw method, and extruded through a 100nm polycarbonate membrane to form liposomes. The prepared liposomes were dialyzed in PBS solution to remove excess doxorubicin hydrochloride.
[0023] b. Formation of gold nanoshells: Take 1mL of the liposome solution prepared above, add 18uL 100nM chloroauric acid solution and 27uL 500nM ascorbic acid solution, the formed gold nanoshells have an absorption peak of 1064nm; In PBS solution, dialyze for 12h.
[0024] c. The connection between siRNA and the gold nanosphere shell: the selected siRNA is green fluorescent protein silencing gene, and the nucleotide sequence of the se...
Embodiment 2
[0026] a. Preparation of cationic liposomes: DPPC, MPPC, DPPE-PEG2000 weight ratio is 90:0:10, add PBS solution, add doxorubicin hydrochloride so that the concentration of doxorubicin hydrochloride is 10nM, and the remaining implementation steps and implementation The preparation process of the cationic liposomes in Example 1 was the same.
[0027] b. The formation of gold nanoshells: the concentrations of chloroauric acid solution and ascorbic acid solution are different, the thickness and particle size of the formed gold shells are all different, and the absorption peaks produced by the plasmon resonance effect are also different. The gold nano shell formed in Example 1 has an absorption peak of 1064nm. In this example, 7uL chloroauric acid solution and 10.5uL ascorbic acid solution are added to form a gold nano shell with an absorption peak of 655nm. The prepared samples were dialyzed again in PBS solution for 12 hours.
[0028] c. The connection between siRNA and the gold...
Embodiment 3
[0030] a. Preparation of cationic liposomes: DPPC, MPPC, DPPE-PEG2000 weight ratio is 90:0:10, and the rest of the implementation steps are the same as the preparation process of cationic liposomes in Example 1.
[0031] b. Formation of gold nanoshells: add 18uL 100nM chloroauric acid solution and 27uL 500nM ascorbic acid solution, and the formed gold nanoshell has an absorption peak of 1064nm; add 7uL chloroauric acid solution and 10.5uL ascorbic acid solution to form gold nanospheres The shell absorption peak is 655nm. The prepared samples were dialyzed again in PBS solution for 12 hours.
[0032]c. Connection of gene and gold nanoshell: Dilute SH-miR-21 (20 μmol / L) with TE buffer (10 mmol / L, Tris-HCL with pH=8.0, 1 mmol / L EDTA) to a certain concentration, The concentration of miR-21 with sulfhydryl groups was 2 μmol / L. According to the pre-designed volume ratio (64:1, 16:1, 2:1), the same amount of SH-miR-21 and gold nanoshells were mixed in TE buffer Mix in the solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com