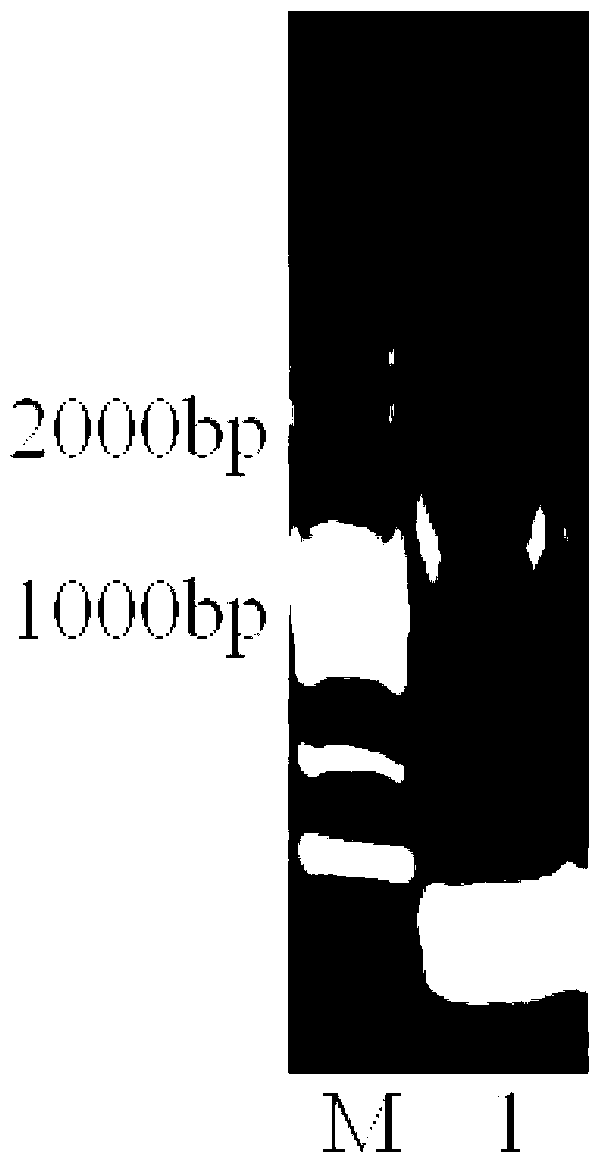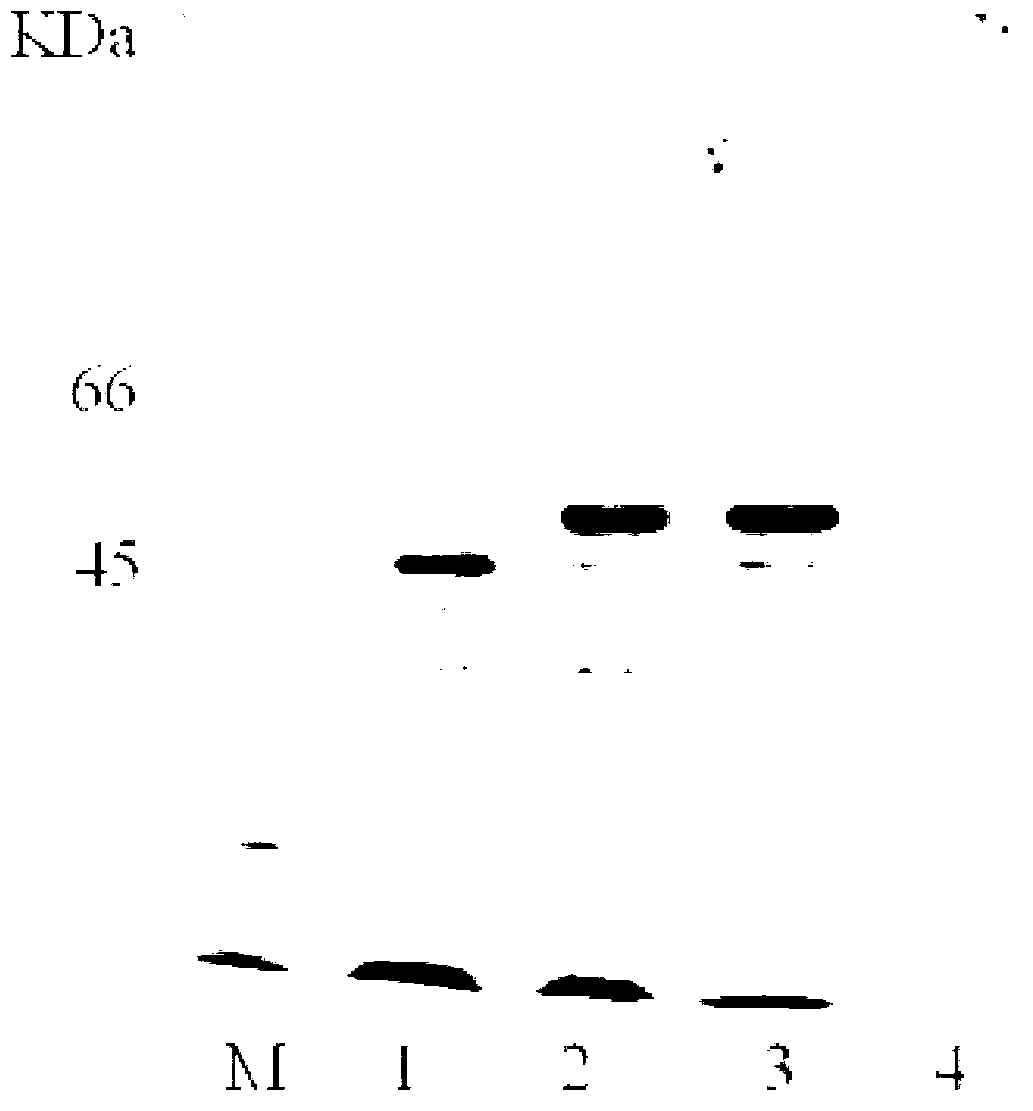(+)Gamma-lactamase, its coding gene and application
A lactamase coding, lactamase technology, applied in the field of enzyme engineering, can solve the problems of poor selectivity, limited industrial application, enzyme instability, etc., and achieves the effects of low cost, high temperature tolerance, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Acquisition of (+) γ-lactamase A-P gene of the present invention
[0040] (1) (+) γ-lactamase A-P genome Japan Bioresource Center (NBRC) direct purchase
[0041] (2) Primer design
[0042] Primers were designed according to the known (+) γ-lactamase A-P gene sequence, the primer sequence is as follows (synthesized by Shanghai Sangong);
[0043] A-P upstream primers:
[0044] 5'-GGAATTC CATATG GTAACAAGGATAACT-3'
[0045] The underline indicates the NdeI restriction site
[0046] A-P downstream primers:
[0047] 5'-CCC AAGCTT CTACTCTGCTAGCTTCTTC-3'
[0048] The underline indicates the HindⅢ restriction site
[0049] (3) PCR amplification and gene cloning
[0050] Use the genomic DNA as a template for PCR amplification. The PCR reaction system is: 1 μL of genomic DNA (100 μg / μL), 2.5 μL of 10×PCR Buffer, 1 μL of upstream and downstream primers, 1 μL of dNTP (2.5 mM, GenStar, Cat#A114 -01), 1mL Taq enzyme (5U / μL, TaKaRa Taq TM , DR001A), with ddH 2 O to ma...
Embodiment 2
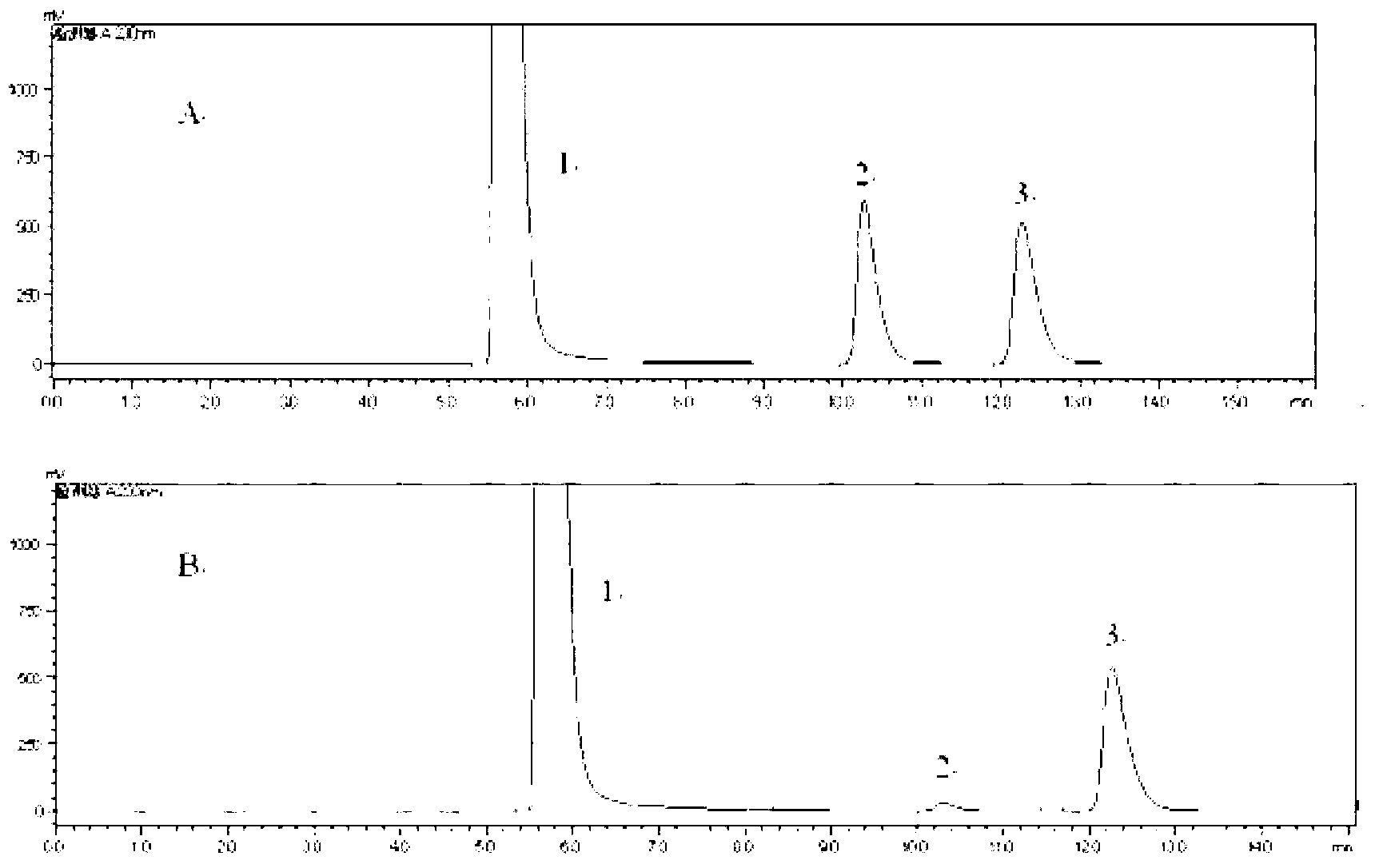
[0060] The quality of the raw materials used in this example is the same as that of Example 1. For the preparation method, refer to Example 1. The improvement is: (+) γ-lactamase is used to resolve the racemic γ-lactam. Take 5 mg of recombinant large intestine Bacillus cells were suspended in 450μL 150mmol pH6.0~8.5 phosphate buffer, 50μL 5g / L (-)γ-lactam substrate was added to the phosphate buffer, and reacted at 60~120℃ for 10~ 24 hours. The above reaction mixture was centrifuged, the supernatant was taken, and 250 μL of ethyl acetate was extracted from the supernatant to obtain the (-)γ-lactam.
[0061] 100 μL of the (-)γ-lactam was taken for HPLC (Shimadzu, Essentia LC-15C) detection, and the optical purity was 91.4%-99.9%, and the yield was greater than 45%.
[0062] The reagents and instruments used in Example 1 and Example 2 are conventional products in the market unless otherwise specified.
[0063] In Example 1 and Example 2, the basic operation methods of genetic e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com