Crosslinkable arylamine compounds and conjugated oligomers of polymers based thereon
A technology of oligomers and polymers, which is applied in the field of electroluminescent devices, can solve the problems of limited charge transport capacity and lack of conjugated polymer skeleton, and achieve the effects of reducing ionization potential, improving charge transport performance, and improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] A) Synthesis of Diphenylbenzocyclobutaneamine (1)
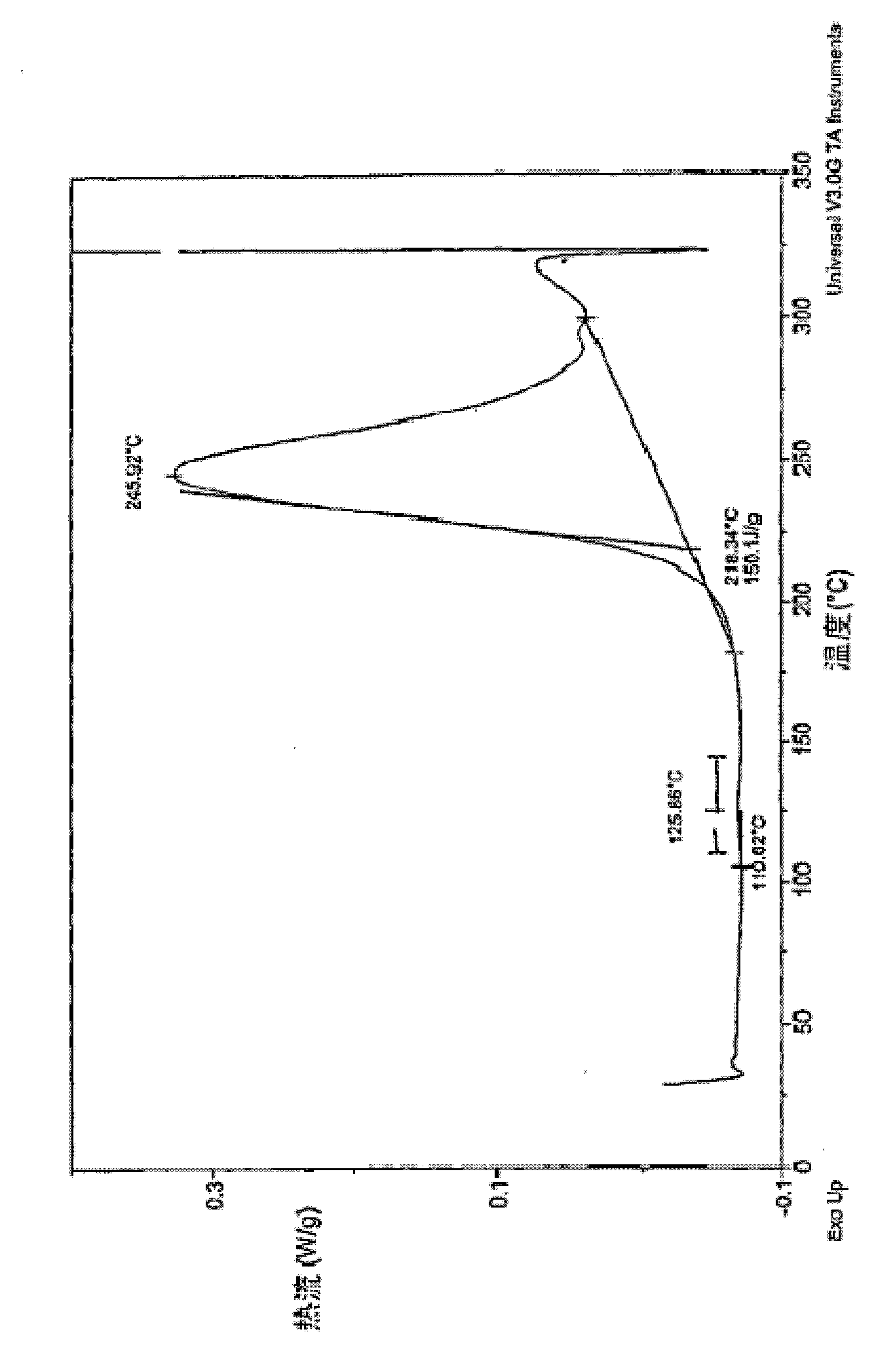
[0164] Into a 500 ml three necked round bottom flask equipped with a mechanical stirrer, nitrogen inlet and reflux condenser (with nitrogen outlet), palladium(II) acetate (196 mg, 1.20 mmol) and tris(ortho-tolyl)phosphine ( 731mg, 2.40mmol) was added to 100ml of toluene. Under nitrogen, the mixture was stirred at room temperature until the palladium catalyst dissolved and the solution turned yellow. Diphenylamine (20.0 g, 118 mmol), bromobenzocyclobutane (23.8 g, 130 mmol) and 400 ml of toluene were added, followed by sodium tert-butoxide (22.8 g, 237 mmol). The reaction mixture turned black by the addition of sodium tert-butoxide. The reaction mixture was heated to reflux under nitrogen for 22 hours. The reaction was quenched by adding 30 ml of 1M aqueous HCl. with 2M Na 2 CO 3 (100 ml) to wash the toluene layer, and then pass the toluene solution over basic alumina. Evaporation of toluene gave a yellow oil. T...
Embodiment 2
[0168] A) Synthesis of fluorene / triarylamine containing BCB(P1) polymer
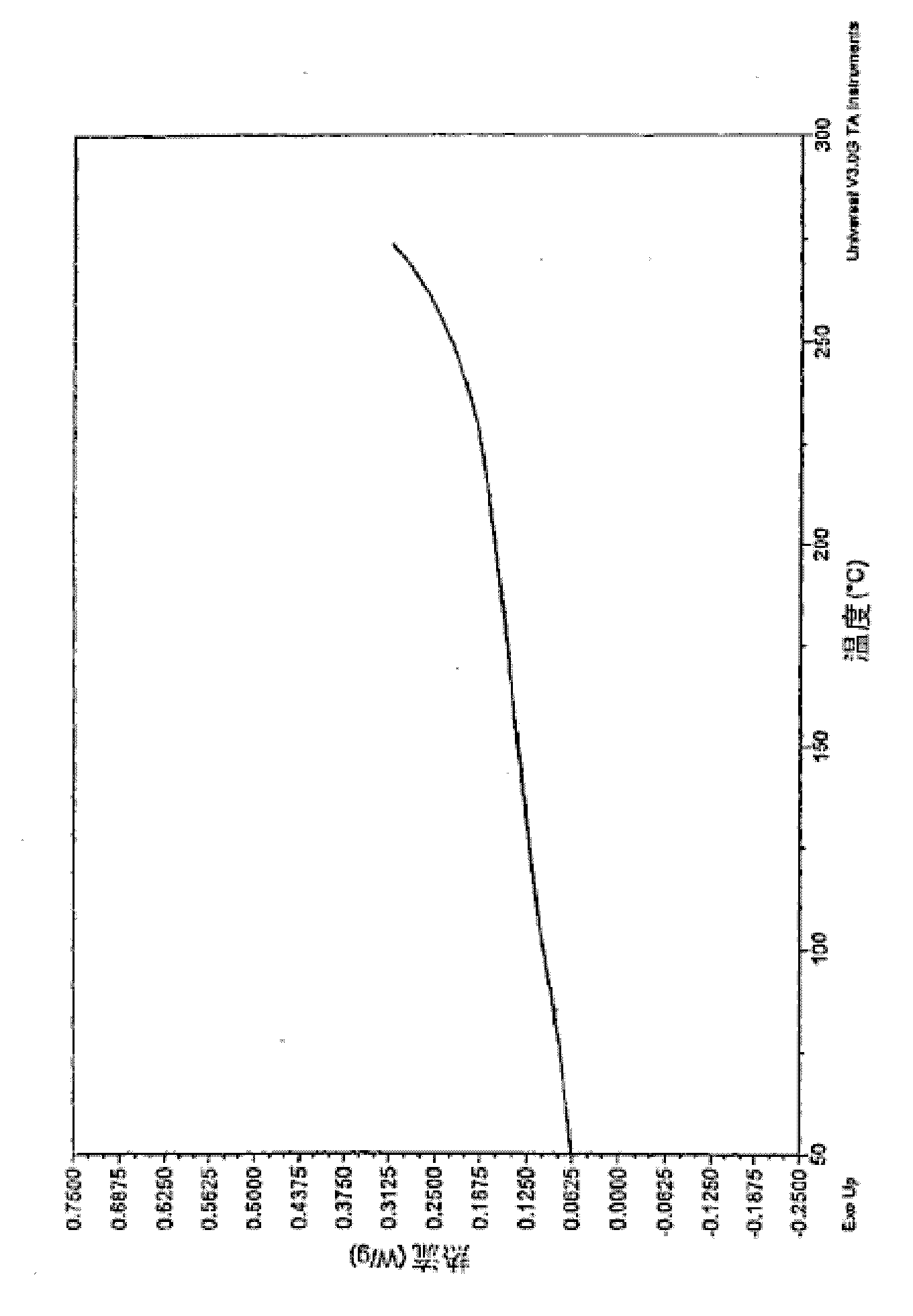
[0169] Into a 1-liter three-neck round bottom flask equipped with a reflux condenser and an overhead stirrer, the following monomers were added: F8BE (3.863 g, 7.283 mmol), TFB (3.177 g, 6.919 mmol) and compound 2 (156.3 mg , 0.364mmol). Add 0.74M quaternary ammonium chloride catalyst (Aliquat TM 336, obtained from Sigma-Aldrich Corporation, 3.1 ml) in toluene, followed by 50 ml of toluene. Add PdCl 2 (PPh 3 ) 2 After the catalyst (4.9 mg), the mixture was stirred in an oil bath (105° C.) until all monomers had dissolved (ca. 15 minutes). Aqueous sodium carbonate (2.0M, 14ml) was added and the reaction mixture was stirred in an oil bath (105°C) for 16.5 hours. Then, phenylboronic acid (0.5 g) was added, and the reaction mixture was stirred for 7 hours. The aqueous layer was removed, and the organic layer was washed with 50 ml of water. The organic layer was returned to the reaction flask, and 0.7...
Embodiment 3
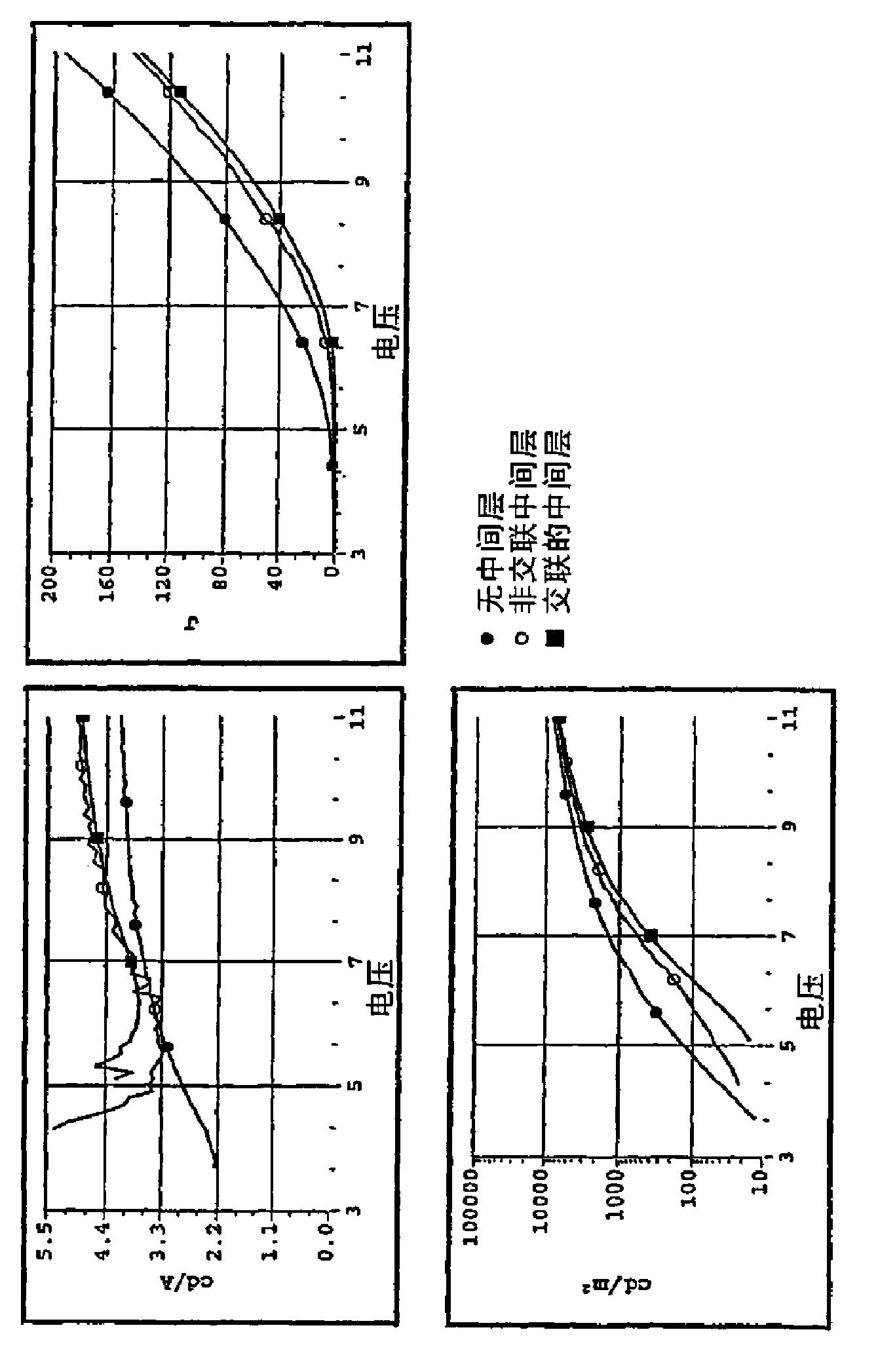
[0171] Scheme 2 shows the synthesis of a phenylenediamine monomer containing a crosslinkable benzocyclobutane group, and the polymerization was used to prepare a fluorene / amine containing 5 mole % of a crosslinkable moiety that would give conjugated crosslinks copolymer.
[0172]
[0173] route 2
[0174] A) Synthesis of N, N'-diphenyl-N, N'-dibenzocyclobutane-1,4-phenylenediamine (3)
[0175] Into a 500 ml three necked round bottom flask equipped with mechanical stirrer, nitrogen inlet and reflux condenser (with nitrogen outlet), palladium(II) acetate (173 mg, 0.80 mmol) and tris(ortho-tolyl)phosphine (486 mg , 1.60mmol) was added to 50ml of toluene. Under nitrogen, the mixture was stirred at room temperature until the palladium catalyst dissolved and the solution turned yellow. Add N,N'-diphenyl-1,4-phenylenediamine (10.0 g, 38.4 mmol), bromobenzocyclobutane (15.5 g, 76.8 mmol) and 200 ml of toluene, followed by sodium tert-butoxide (7.37 g, 76.8 mmol). The reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com