Anthraquinone derivative material and preparation method and application thereof
An anthraquinone derivative and compound technology, applied in the field of anthraquinone derivative materials and their preparation, can solve the problems of inability to form high-quality thin films, pinholes, poor thermal stability, etc., and achieve excellent structural stability and excellent thermal stability. performance, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
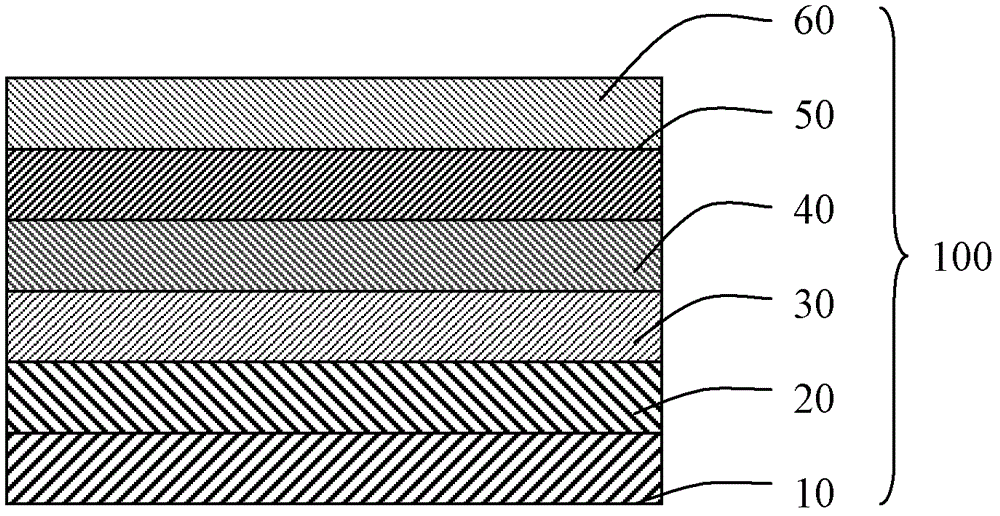
Image
Examples
preparation example Construction
[0041] A preparation method of the above-mentioned anthraquinone derivative material P, comprising the following steps:
[0042] Step S1, providing compound A represented by the following structural formula, and boronic acid or boronic acid ester corresponding to Ar.
[0043] A is: Wherein X is Cl or Br, preferably Br.
[0044] The boric acid corresponding to Ar is:
[0045]
[0046] The boronate corresponding to Ar is:
[0047]
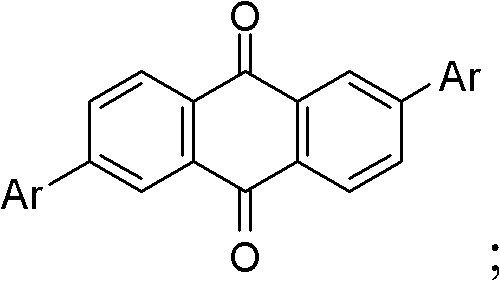
[0048] Among them, -Ar is:
[0049]
[0050] Step S2, dissolving the boric acid or boric acid ester corresponding to compound A and Ar in an organic solvent at a molar ratio of 1:2 to 1:4, then adding a catalyst, and reacting at 70°C to 130°C for 10 hours to 48 hours to obtain Anthraquinone derivative material represented by the following structural formula:
[0051]
[0052] The overall reaction equation is as follows:
[0053]
[0054] The reaction temperature is preferably 80°C to 100°C.
[0055] The molar ratio of compound ...
Embodiment 1
[0061] The present embodiment prepares 2,6-dipyrene-9, and the reaction process of 10-anthraquinone is as follows:
[0062]
[0063] Above-mentioned 2,6-dipyrene-9, the preparation of 10-anthraquinone is as follows:
[0064] Under nitrogen protection, 2,6-dibromo-9,10-anthraquinone (3.29g, 9mmol), pyreneboronic acid (5.76g, 23.4mmol) and tetrakistriphenylphosphine palladium (Pd(PPh 3 ) 4) (208mg, 0.18mmol) was dissolved in 80ml of benzene, and then sodium carbonate solution (2.0mol / L, 90mL) was added. The mixture was vigorously stirred and reacted at 110°C for 10 h, then cooled to room temperature, extracted three times with dichloromethane, the organic phases were combined, washed with 1 mol / L sodium hydroxide solution, dried with anhydrous magnesium sulfate and then spin-dried, and the crude product was obtained by petroleum ether The volume ratio of ethyl acetate to 10:1 was used as eluent and separated by silica gel chromatography to obtain a white solid with a yield ...
Embodiment 2
[0071] The present embodiment prepares 2,6-two (10'-(9'-phenyl)anthracene)-9, the reaction process of 10-anthraquinone is as follows:
[0072]
[0073] The preparation of above-mentioned 2,6-two (10'-(9'-phenyl)anthracene)-9,10-anthraquinone is as follows:
[0074] Under nitrogen protection, 2,6-dibromo-9,10-anthraquinone (3.29g, 9mmol), 9-phenyl-10-anthraceneboronic acid (5.76g, 23.4mmol) and bistriphenylphosphine dichloride Palladium chloride (315.9 mg, 0.45 mmol) was dissolved in 80 ml of ethylene glycol dimethyl ether, and then potassium hydroxide solution (2.0 mol / L, 90 mL) was added. The mixture was vigorously stirred and reacted at 70°C for 36 hours, then cooled to room temperature, extracted three times with dichloromethane, combined organic phases, washed with 1mol / L sodium hydroxide solution, dried over anhydrous magnesium sulfate and then spin-dried, and the crude product was obtained by petroleum ether The volume ratio of ethyl acetate to 10:1 was used as the e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| light emitting area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com