2,3-diaryl thiazolidinone compound and analogue, and purpose thereof in preparing antiangiogenic drugs
A technology based on thiazolinone and thiazolinone, which is applied in the field of 2,3-diarylthiazolinone compounds and analogs, can solve the problems of easy recurrence, large side effects, genetic instability of cancer cells and easy mutation, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1-12-(3-bromophenyl)-3-(2-methoxyphenyl)-4-thiazolinone
Embodiment 1-1
[0127] Take the compound 3-bromobenzaldehyde (185mg, 1.0mmol) and o-methoxyaniline (113μL, 1.0mmol) in toluene, under a nitrogen atmosphere, stir in an ice bath for 5min, then change to an oil bath and heat to reflux, The device is connected with an oil-water separator. When anhydrous is produced, cool down after completion, add the compound thioglycolic acid (70 μL, 1.0 mmol) and continue heating to reflux. After the water separation is completed, the crude product is obtained. After purification by column chromatography, the compound AGT001 (309 mg, yield: 85% ): 1 H NMR(400MHz,DMSO)δ7.52(s,1H),7.34(d,J=8.0Hz,1H),7.19(dd,J=8.0,8.0Hz,1H),7.09(dd,J=8.0, 8.0Hz,1H),6.92(d,J=8.0Hz,1H),6.87-6.80(m,2H),6.02(s,1H),3.94(AB,J=15.6Hz,1H),3.86(AB, J=15.6Hz,1H),3.81(s,3H). 13 C NMR (100MHz, CDCl 3 )δ 171.3, 154.9, 141.7, 132.1, 130.9, 130.1, 130.0, 129.8, 126.5, 125.3, 122.5, 121.0, 112.1, 64.1, 55.8, 33.1.
[0128] The preparation of AGT002-AGT067 thiazolinone compounds shown in Ex...
Embodiment 2
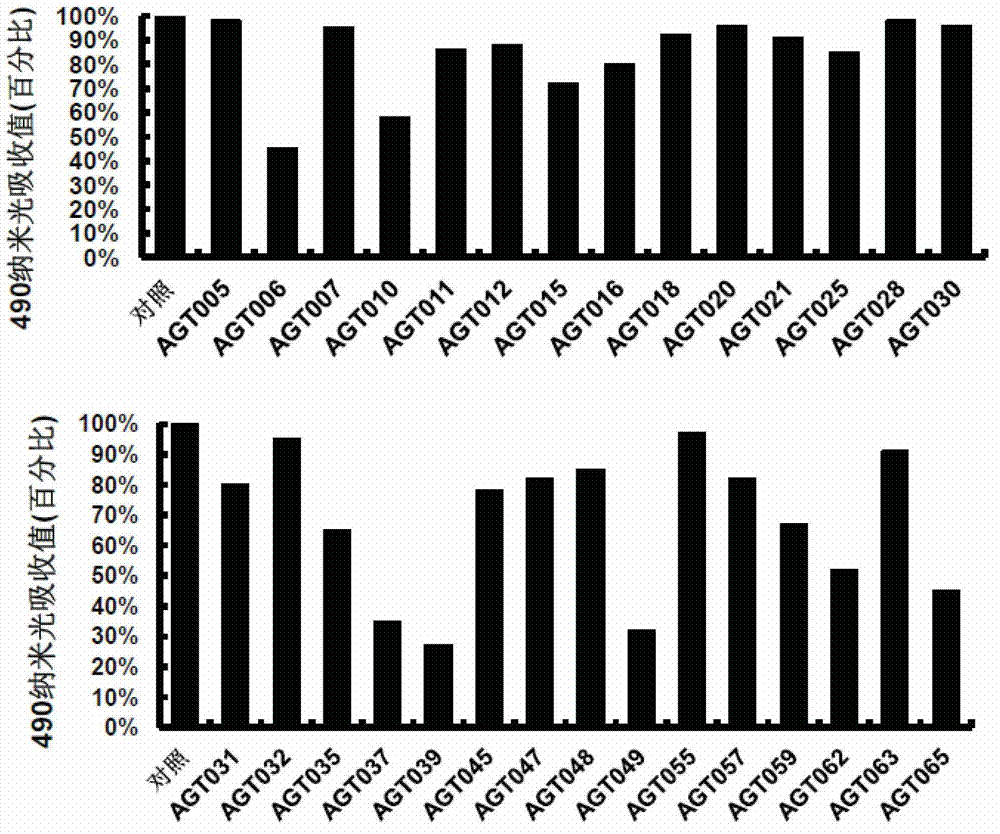
[0140] Purpose and principle: MTS assay was used in the cell proliferation experiment. The MTS assay is an assay that uses a colorimetric method to determine the number of cell proliferation in living cells. NADPH dehydrogenase converts MTS tetrazolium salt compound into a colored substance - formazan, which is soluble in tissue culture medium, and its color depth is highly correlated with the number of living cells within a certain range. This model can be used to evaluate the effect of drugs on cell proliferation.
[0141] Method: Human umbilical vein endothelial cells were added to the 96-well plate, and the control group and sample addition group were set up. The sample addition group was added with the compound to be tested at a final concentration of 40 micromolar. After incubation for 60-72 hours, the cell proliferation detection reagent was added and incubated. After 1-3 hours, detect the light absorbance at 490 nm with a microplate reader.
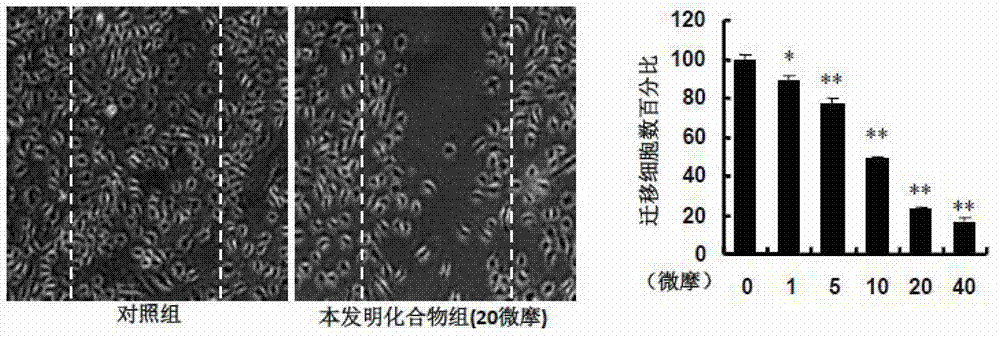
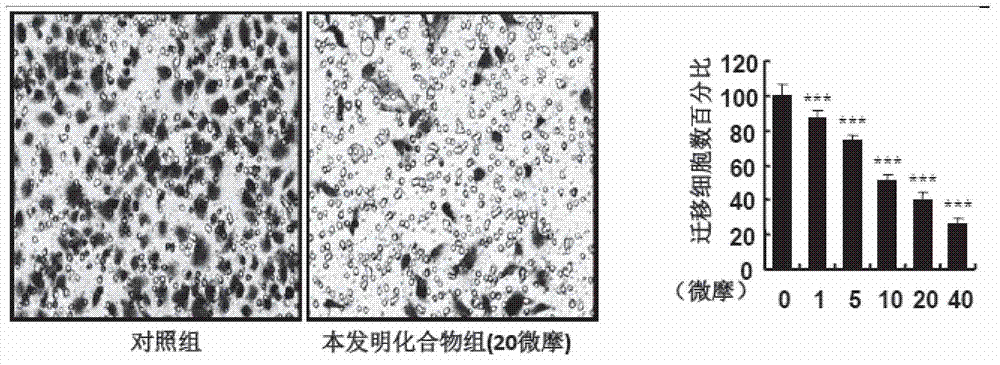
[0142] Results and evalu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com