Method for chiral separation and measurement of methyl lactate optical isomers by capillary gas chromatography
A technology of optical isomers and gas chromatography, which is applied in the field of chiral separation and determination of methyl lactate optical isomers by capillary gas chromatography, can solve the problems of the influence of detection results and the inapplicability of target detection substances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
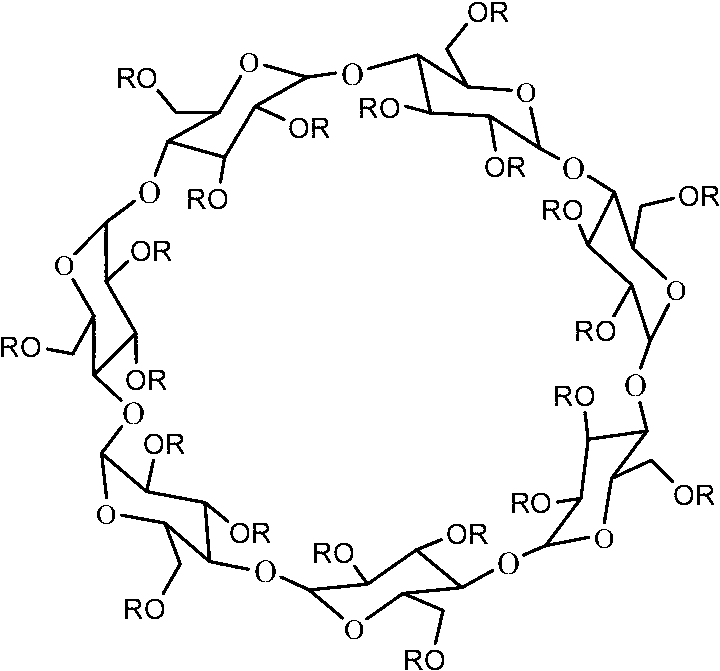
[0032] Example 1: Preparation of 2,3,6-tri-O-octanoyl-β-cyclodextrin (CSP2)
[0033] Dissolve 0.3g (0.264mmol) of β-cyclodextrin in 15mL of dry chloroform, add 2.5mL of pyridine at 0°C, add 3.2mL of octanoyl chloride (21.06mmol) dropwise under ice-cooling, and stir at 0°C for 0.5h. The reaction was stirred at 60°C for 6h. After the reaction was completed, the solvent was removed under reduced pressure, dissolved in 20 mL of ice water, extracted three times with 20 mL of chloroform, the chloroform layers were combined, washed twice with saturated aqueous sodium bicarbonate solution, then washed twice with water, and finally dried over anhydrous sodium sulfate , concentrated under reduced pressure to obtain a crude product. Purification by column chromatography (toluene / ethyl acetate=1:1, v / v) gave a brown viscous product (yield 20%).
[0034] The structural confirmation data are as follows:
[0035] IR (cm -1 ): 2927, 2857 ((CH 2 , CH 3 )), 1746(C=O), 1462, 1377(CH 2 , C...
Embodiment 2
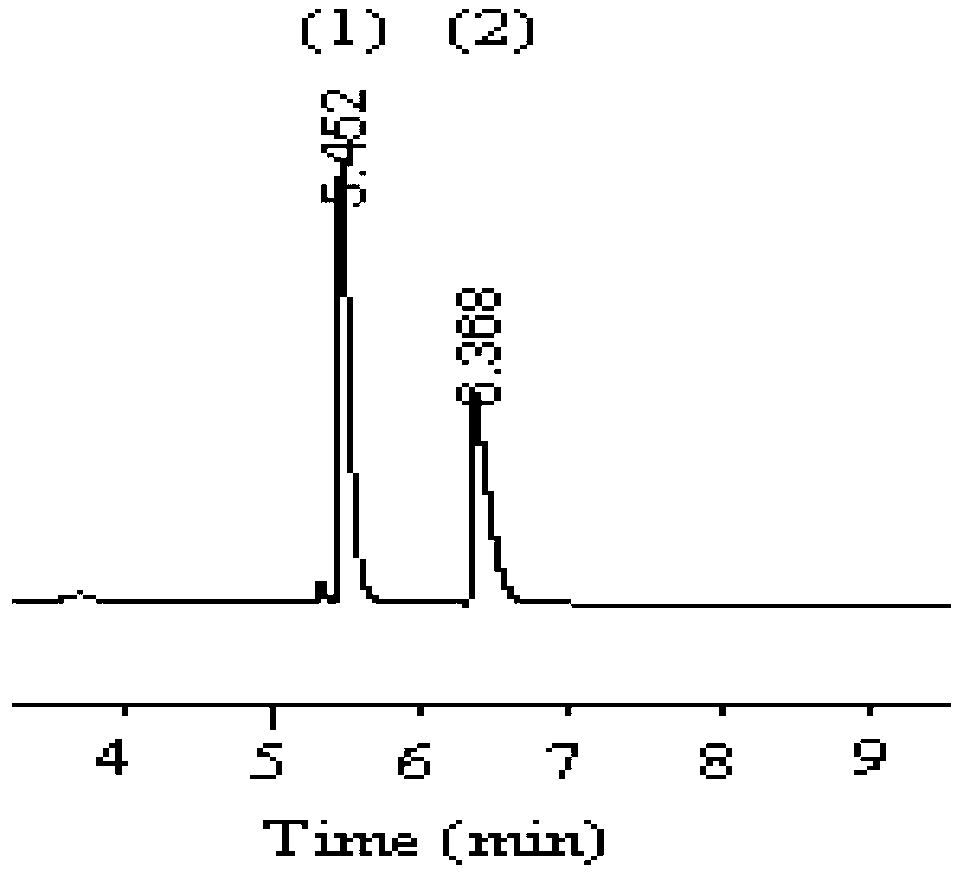
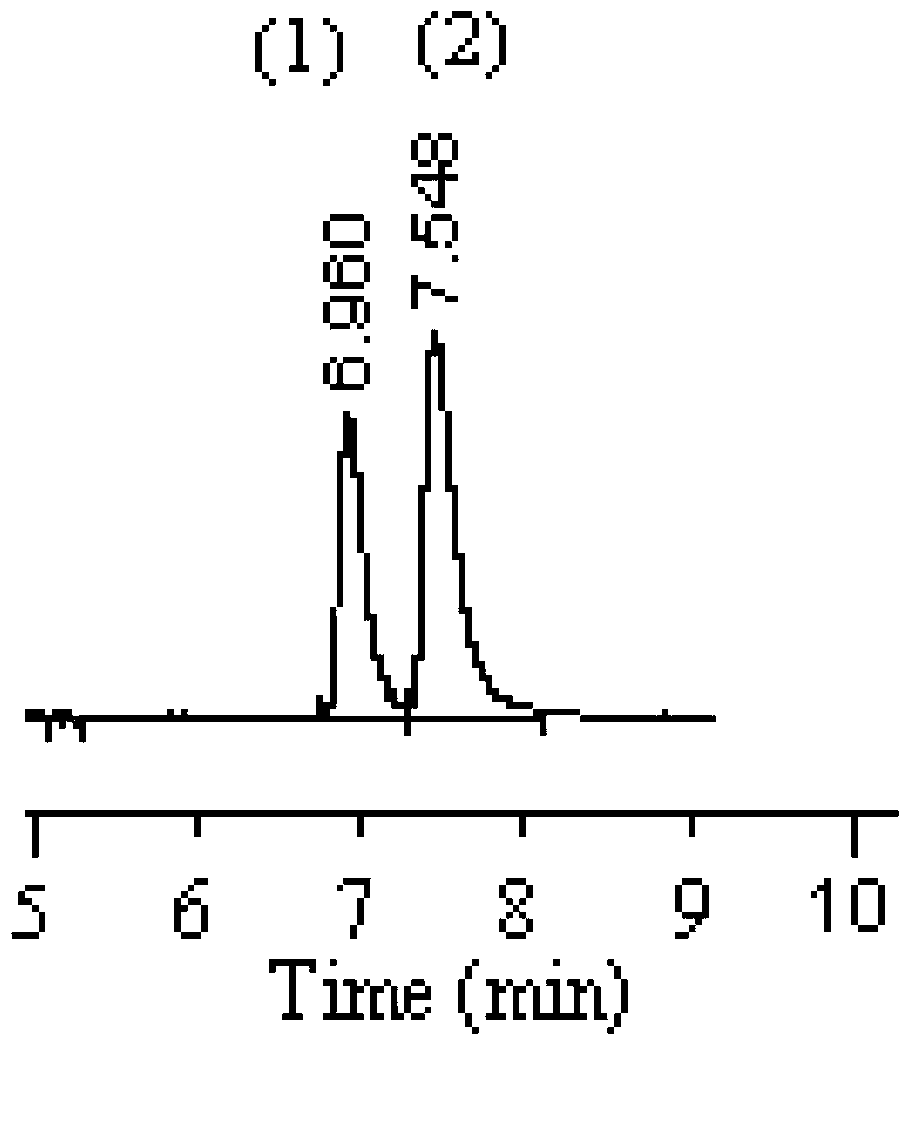
[0038] Embodiment 2, adopt capillary gas chromatography to carry out the separation of methyl lactate D, L optical isomers
[0039] 1) Preparation of capillary gas chromatography chiral column
[0040] The 2,3,6-tri-O-octanoyl-β-cyclodextrin prepared in Example 1 was used as the chiral stationary phase, and after being dissolved in dichloromethane, the wall of the stationary phase was coated on the The inner wall of the quartz capillary column was roughened with NaCl to prepare a capillary gas chromatography chiral column, and the inner wall thickness of 2,3,6-tri-O-octanoyl-β-cyclodextrin was 0.31 μm.
[0041] 2) Chromatographic conditions
[0042] Instrument: HP6890 + Gas Chromatograph, Hydrogen Flame Ionization Detector, Split / Splitless Inlet
[0043]Chromatographic column: a self-made capillary chiral column (20m × 0.25mm × 0.31 μm, long × inner diameter × inner wall thickness);
[0044] Mobile phase: high-purity nitrogen
[0045] Mobile phase flow rate: 29cm / s;
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com