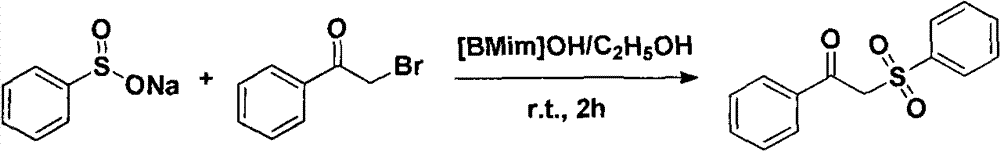Novel method for preparing 1-phenyl-2-benzene sulfonyl acetophenone
A technology of benzenesulfonyl ethyl ketone and bromoacetophenone, applied in the field of preparation of organic synthesis intermediates, can solve the problems of medium yield, high reaction temperature, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
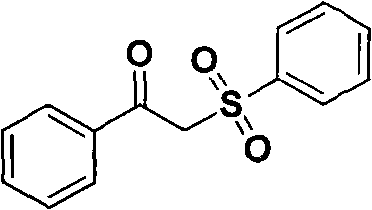
[0014] Example 1. Synthesis of 1-phenyl-2-benzenesulfonyl ethanone, the structural formula is as follows:
[0015]
[0016] Add 1.5mmol (246.2mg) of raw material sodium benzenesulfinate and 1.0mL of 50% ethanol aqueous solution into a 25ml round bottom flask, dissolve the raw materials under stirring conditions, and then add 1.0mmol (199.0) of 2-bromoacetophenone mg), and 0.2mmol (31.25mg) 1-butyl-3-methylimidazole hydroxide ([Bmim]OH) alkaline ionic liquid (structure: ) As a catalyst, the reaction is stirred at room temperature for 2 hours. After the completion of the reaction is detected by TLC, the reaction is stopped, and the crude product is obtained by suction filtration. The crude product was recrystallized with absolute ethanol to obtain 212.2 mg of pure white solid 1-phenyl-2-benzenesulfonyl ethanone with a purity of 98.6%. The isolated yield was 81.5% and the melting point was 93-94°C.
[0017] Identification of 1-phenyl-2-benzenesulfonyl ethyl ketone:
[0018] Infrared s...
Embodiment 2
[0022] In a 25ml round-bottom flask, add 1.2mmol (197.0mg) of raw material sodium benzenesulfinate and 1.0mL of 50% ethanol aqueous solution, dissolve the raw materials under stirring, and then add 1.0mmol (199.0) of 2-bromoacetophenone mg), and 0.2mmol (31.25mg) 1-butyl-3-methylimidazole hydroxide ([Bmim]OH) alkaline ionic liquid as a catalyst. The reaction was stirred at room temperature for 2 hours. After the reaction was completed by TLC, the reaction was stopped. reaction. Suction filtration to obtain a crude product. The crude product was recrystallized with absolute ethanol to obtain 204.6 mg of pure white solid 1-phenyl-2-benzenesulfonyl ethanone with a purity of 98.5%. The isolated yield was 78.6% and the melting point was 93-94°C.
[0023] The identification of the product is consistent with the literature report.
Embodiment 3
[0025] In a 25ml round-bottomed flask, 1.0mmol (164.16mg) of sodium benzenesulfinate as raw material and 1.0mL of 50% ethanol aqueous solution were added, and the raw materials were dissolved under stirring, and then 1.0mmol (199.0) of 2-bromoacetophenone was added. mg), and 0.2mmol (31.25mg) 1-butyl-3-methylimidazole hydroxide ([Bmim]OH) alkaline ionic liquid as a catalyst. The reaction was stirred at room temperature for 2 hours. After the reaction was completed by TLC, the reaction was stopped. reaction. Suction filtration to obtain a crude product. The crude product was recrystallized with absolute ethanol to obtain 177.8 mg of pure white solid 1-phenyl-2-benzenesulfonyl ethanone with a purity of 98.5%. The isolated yield was 68.3% and the melting point was 93-94°C.
[0026] The identification of the product is consistent with the literature report.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com