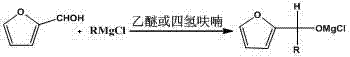Synthesis method of ethyl maltol intermediate
A technology of ethyl maltol and a synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of easy volatility, high toxicity to the operator's body, and many losses, so as to reduce production and discharge, improve atomic utilization, and reduce production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
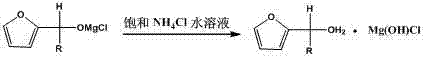
[0026] Example 1: Magnesium powder (0.60 g, 25 mmol), iodine (0.06 g, 0.24 mmol), bromoethane (0.27 g, 2.5 mmol) were added to a 100 mL three-necked flask, and 30 mL of 2- Methyltetrahydrofuran, heated until the yellow color of the system faded, and passed through ethyl chloride gas (1.76 g, 27.5 mmol), the temperature was maintained at 20 ° C, and the reaction was completed for 60 minutes. The Grignard reagent was prepared; Furfural (1.92 g, 20 mmol), remove the water bath after the dropwise addition, stir at room temperature for 4 hours, monitor the end of the reaction, and use saturated NH 4 Cl extraction was performed three times (10 mL*3), and the solvent was removed by rotary evaporation to obtain the crude furfuryl ethanol, which was distilled under reduced pressure to obtain 2.06 g furfuryl ethanol (62-63°C / 8 mmg, yield 81.7%). The product was characterized by H NMR spectrum, which was consistent with the literature value. 1 H NMR (400 MHz, CDCl 3 ):δ 6.27 (dd, J =...
Embodiment 2
[0027] Example 2: Magnesium powder (0.60 g, 25 mmol), iodine (0.12 g, 0.47 mmol), bromoethane (0.27 g, 2.5 mmol) were added to a 100 mL three-necked flask, and 30 mL of 2- Methyl tetrahydrofuran, heated until the yellow color of the system faded, and passed through ethyl chloride gas (1.76 g, 27.5 mmol), the temperature was maintained at 30 ° C, and the reaction was completed for 30 minutes. The Grignard reagent was prepared; Furfural (1.92 g, 20 mmol), remove the water bath after the dropwise addition, stir at room temperature for 4 hours, monitor the end of the reaction, and use saturated NH 4 Cl extraction was performed three times (10 mL*3), and the solvent was removed by rotary evaporation to obtain the crude furfuryl ethanol, which was distilled under reduced pressure to obtain 2.08 g furfuryl ethanol (62-63°C / 8 mmg, yield 82.5%).
Embodiment 3
[0028] Example 3: Magnesium powder (0.60 g, 25 mmol), iodine (0.24 g, 0.95 mmol), bromoethane (0.27 g, 2.5 mmol) were added to a 100 mL three-necked flask, and 30 mL of 2- Methyltetrahydrofuran, heated until the yellow color of the system faded, and chloroethane gas (1.76 g, 27.5 mmol), the temperature was maintained at 40 ° C, the reaction was 120 minutes, and the Grignard reagent was prepared; add dropwise to the Grignard reagent under ice bath Furfural (1.92 g, 20 mmol), remove the water bath after the dropwise addition, stir at room temperature for 4 hours, monitor the end of the reaction, and use saturated NH 4 Cl extraction was performed three times (10 mL*3), and the solvent was removed by rotary evaporation to obtain the crude furfuryl ethanol, which was distilled under reduced pressure to obtain 2.05 g furfuryl ethanol (62-63°C / 8 mmg, yield 81.3%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com