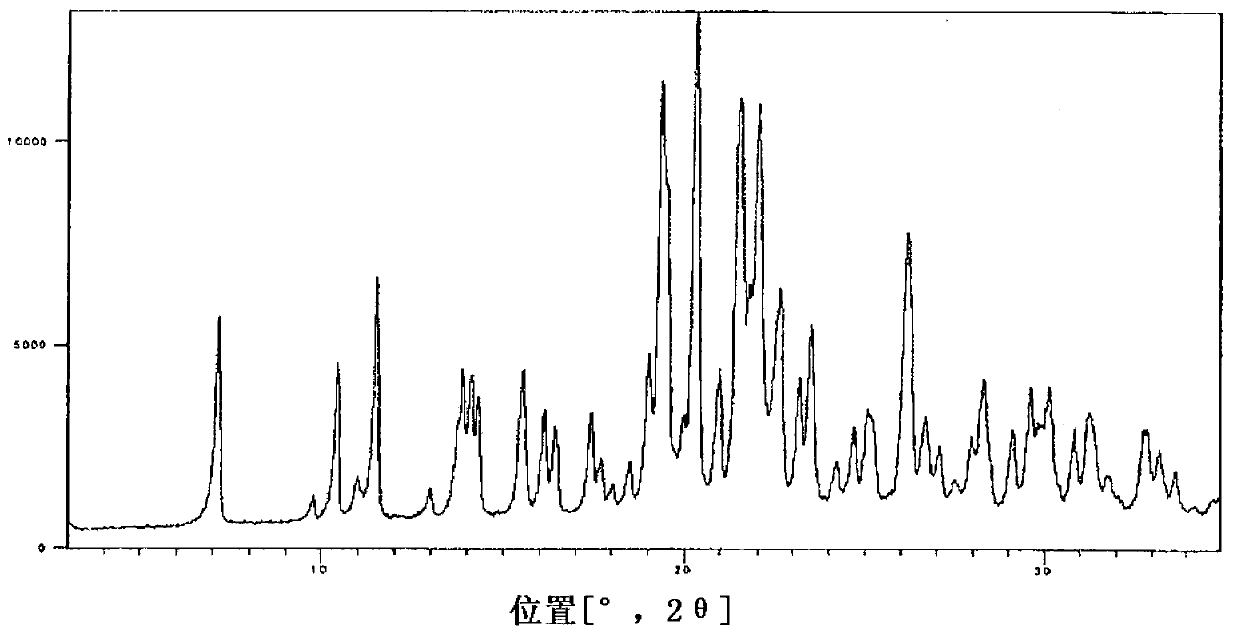
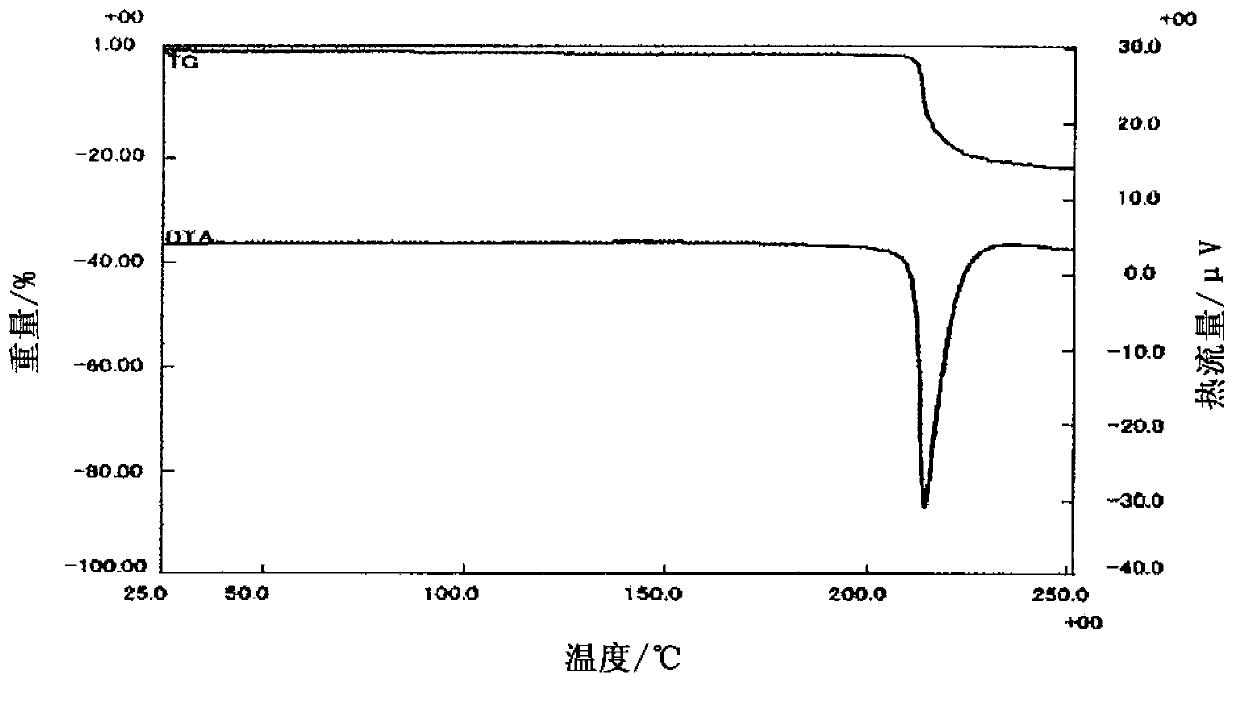
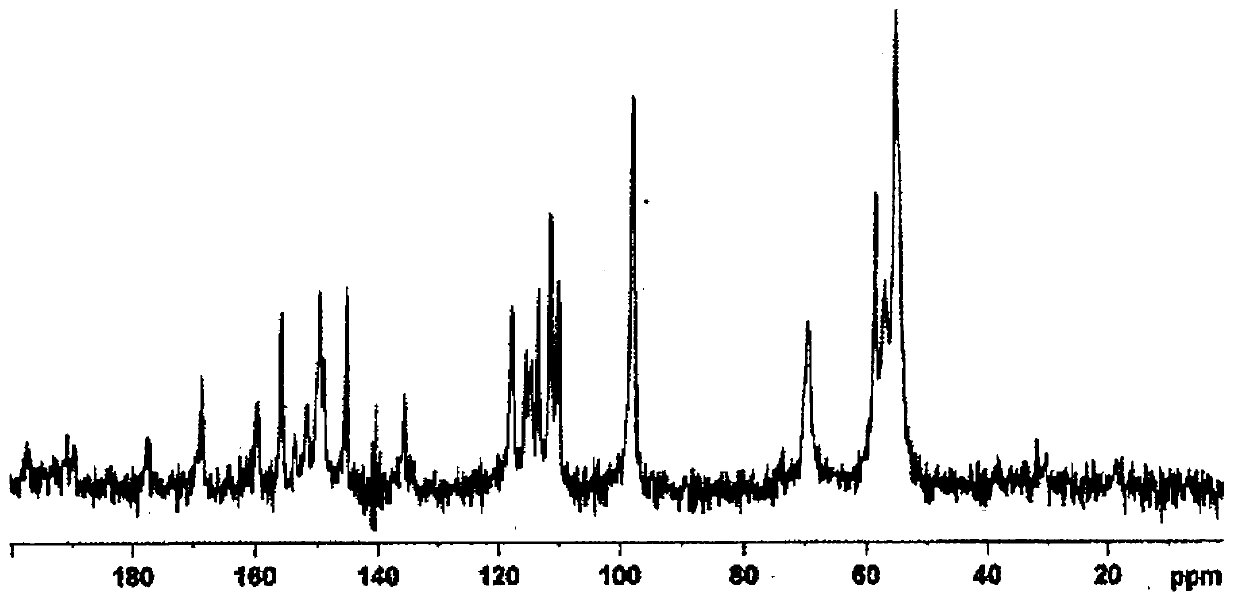
Salt of fused heterocyclic derivative and crystal thereof
A technology of crystals and compounds, applied in the field of salts of fused heterocyclic derivatives and their crystals, can solve the problems of no reports, etc., and achieve the effects of easy handling, good storage stability and fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Compound (A)
[0040] The 3-[2-fluoro-5-(2,3-difluoro-6-methoxybenzyloxy)-4-methoxyphenyl]-2,4-dioxo-1,2,3 ,4-Tetrahydrothieno[3,4-d]pyrimidine-5-carboxylic acid (3.07g) and 46% choline hydroxide aqueous solution (1.64g) are suspended in 1-propanol and water (about 1:1 volume ratio; 30mL) in the mixed solution, and the mixture was heated at 60°C and stirred for 15 minutes. To the mixture was added 1-propanol (30 mL) at 60°C, and the mixture was stirred at room temperature for 1 hour, and then stirred for another hour under ice cooling. After collecting the precipitate by filtration, it was washed twice with 1-propanol (1 mL). The obtained solid was dried under reduced pressure at 40° C. to obtain compound (A) (2.93 g). In addition, the compound was heated and stirred at 60° C. in a mixed solvent of 1-propanol and water (about 1:1 volume ratio; 30 mL), and 1-propanol (30 mL) was added to the solution obtained after hot filtration. The mixture was cooled to room temperat...
Embodiment 2
[0056] Compound (A)
[0057] The 3-[2-fluoro-5-(2,3-difluoro-6-methoxybenzyloxy)-4-methoxyphenyl]-2,4-dioxo-1,2,3 ,4-Tetrahydrothieno[3,4-d]pyrimidine-5-carboxylic acid (4.07g), 46% choline hydroxide aqueous solution (2.18g), 2-propanol (200mL) and water (100mL) are mixed, And heated at 40°C for 15 minutes. After the insoluble matter was removed by filtration, the resulting solution was concentrated and 2-propanol (80 mL) and diisopropyl ether (80 mL) were added. Then, the mixture was stirred at room temperature for about 1 hour, and then stirred under ice cooling for about 4 hours. The precipitated solid was collected and dried under reduced pressure at 90° C. overnight (yield 3.59 g). Then a mixed solution of ethanol and water (about 1:1 volume ratio; 25 mL) was added to the obtained solid (3.56 g). The mixture was heated to 65°C, and after adding a mixed solution of ethanol and water (1:1 volume ratio; 10 mL) at the same temperature, it was subjected to hot filtration. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com