Drug with characteristics of rapid transaminase lowering and live protection
A transaminase and drug technology, applied in the direction of drug combination, pharmaceutical formula, plant raw materials, etc., to achieve high cure rate, high safety, and rapid effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] The present invention is produced by Jiangsu Yihai Pharmaceutical Co., Ltd. and adopts the conventional process of traditional Chinese medicine preparation to make capsules. 0.45g per capsule.
experiment example 1
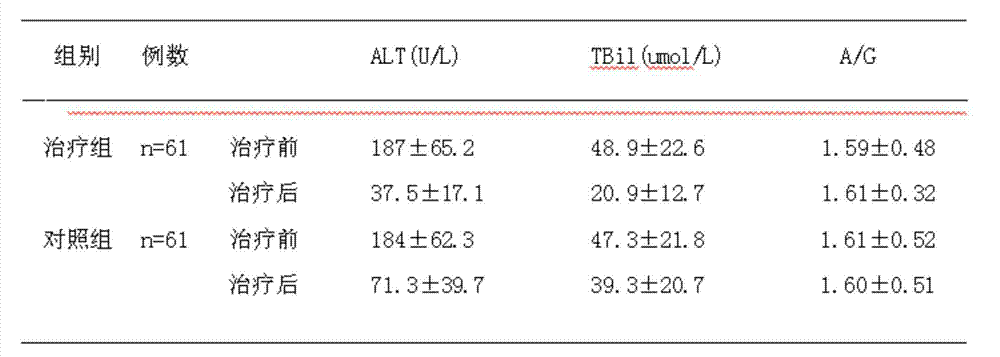
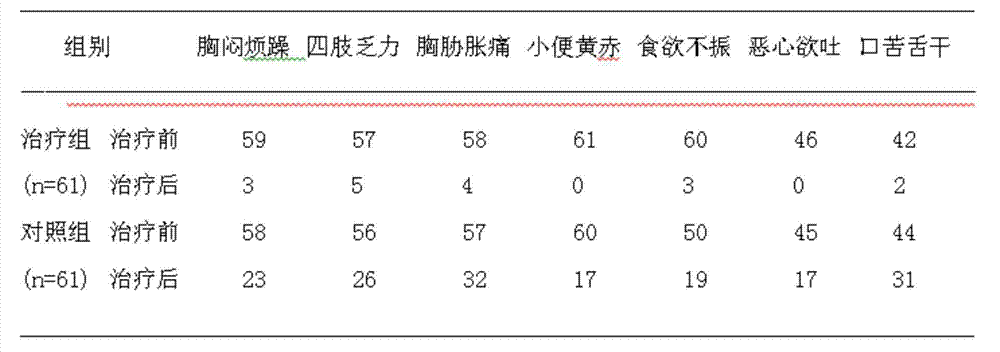
[0035] Clinical experiment example 1: for the treatment of elevated transaminases.
[0036] normal information
[0037] A total of 122 cases of patients with elevated transaminases were observed, and both groups of patients were from outpatient clinics. The diagnoses of all cases of patients conformed to the diagnostic criteria established by the 2000 (Xi'an) National Conference on Infectious Diseases.
[0038] Randomly divided into a treatment group and a control group, 61 cases in the treatment group, 46 males and 15 females; 61 cases in the control group, 45 males and 16 females; the disease course of the two groups was half a year to 3.5 years, and the average age was 37±8 years old; There was no history of strong enzyme-lowering drugs in the two groups within 3 months before treatment.
[0039] treatment method
[0040] Treatment group: oral administration of drug capsules of the present invention (produced by Jiangsu Yihai Pharmaceutical Co., Ltd.), 4 capsules (0.45 g...
experiment example 2
[0053] Clinical trial example 2: for non-alcoholic fatty liver (NAFL)
[0054] A total of 106 patients with NAFL were observed. The effect of treatment was observed by self-control method. All patients were from outpatient clinics. Among them, there were 75 males and 31 females; the age was (39±8) years old; the course of disease was 1 year to 12 years and 6 months; 79 cases were obese, with an average body mass index of 27.86.
[0055] Therapeutic method: all patients take the drug capsules of the present invention (produced by Jiangsu Yihai Pharmaceutical Co., Ltd.), 4 capsules (0.45g each) / time, 3 times / day. Treatment for 3 months is a course of treatment, and the observation indicators are re-examined to determine the curative effect.
[0056] Diagnostic criteria: According to the "Diagnostic Criteria for Non-alcoholic Fatty Liver (Draft)" formulated by the Fatty Liver and Alcoholic Liver Disease Group of the Hepatology Society of the Chinese Medical Association, anyone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com