Substituted tricarbonyl chloride 2, 2', 4, 2'-terpyridyl rhenium (I) coordination compound, preparation method and application thereof
A technology for chlorinating tricarbonyl and terpyridine, which is applied in the field of organic electroluminescence devices, can solve the problems of annihilation, unachieved carrier transport performance, long excited state lifetime, etc., and achieves stable properties, high yield, and improved performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Ligand Preparation and Structure Verification
[0028] The ligands are: 4-carbazole-2,2',4,2'-terpyridine (L1), 4-dianilino-2,2',4,2'-terpyridine (L2), 4- Bis(4-tert-butyl)anilino-2,2',4,2'-terpyridine (L3), 4-(N-phenyl-1-naphthalene)amino-2,2',4,2' -Terpyridine (L4), 4-(N-phenyl-2-naphthalene)amino-2,2',4,2'-terpyridine (L5).
[0029] 1. The preparation method of the ligand: in a reaction kettle, carbazole, diphenylamine, two (4-tert-butyl) aniline, (N phenyl-1-naphthalene) amine or (N phenyl-2-naphthalene) amine ( 3.0 mmol) with 4-Cl-2,2',4,2'-tripyridine (0.22 g, 0.82 mmol), potassium tert-butoxide (0.22 g, 2.0 mmol) and 1,3-dimethyl-3, 4,5,6-tetrahydro-2(1H)-pyrimidinedione (DMPU) (0.1 mL) mixed, under pure nitrogen atmosphere, at 205 o C reacted for 10-15 hours; after the reaction, the reaction mixture was cooled to 20 o C-25 oC , to extract the ligand 4-carbazole-2,2',4,2'-terpyridine (code L1, to obtain 0.12 g, yield: 39%) or diphenylamine-2,2',...
Embodiment 2
[0064] Example 2: Preparation and structural verification of complexes
[0065]The complexes are tricarbonyl chloride (4-carbazole-2,2',4,2'-terpyridine) rhenium (I) (code named as Re1), tricarbonyl chloride (4-diphenylamino-2 ,2',4,2'-terpyridine) rhenium (I) (code named Re2), tricarbonyl chloride (4-bis(4-tert-butyl)anilino-2,2',4,2 '-terpyridine) rhenium (I) (code named Re3), tricarbonyl chloride (4-(N-phenyl-1-naphthalene) amino-2,2',4,2'-terpyridine) Rhenium (I) (code name is Re4), tricarbonyl chloride (4-(N-phenyl-2-naphthalene) amino-2,2',4,2'-terpyridine) rhenium (I) (code name Set to Re5).
[0066] 1. Preparation of complexes
[0067] Each ligand is mixed with rhenium pentacarbonyl chloride (i.e. (Re(CO) 5 Cl) were mixed according to equimolar ratio (0.10 mmol), heated to 110 in anhydrous toluene (30 mL) o C reflux reaction for 6 hours, after the reaction finished, the solvent in the reaction mixture was distilled off, and the obtained yellow solid was purified ...
Embodiment 3
[0101] Example 3 Fluorescent Characterization of Complexes
[0102] Complexes include: tricarbonyl chloride (4-carbazole-2,2',4,2'-terpyridine) rhenium (I) Re1), tricarbonyl chloride (4-dianilino-2,2' ,4,2'-terpyridine) rhenium(I) (Re2), tricarbonyl chloride (4-di(4-tert-butyl)anilino-2,2',4,2'-terpyridine) Rhenium(I)(Re3), tricarbonyl chloride (4-(Nphenyl-1-naphthalene)amino-2,2',4,2'-terpyridine) rhenium(I)(Re4) and chlorine Tricarbonyl (4-(N-phenyl-2-naphthalene)amino-2,2',4,2'-terpyridine) rhenium(I)(Re5).
[0103] Using dichloromethane as a solvent, the above-mentioned complexes of rhenium were made into concentrations of 10 -3 M's solution. At room temperature, the following data of each complex were determined separately:
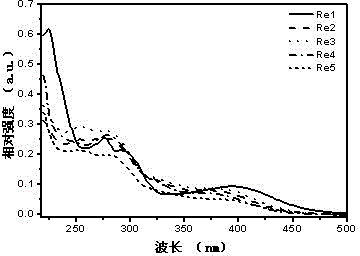
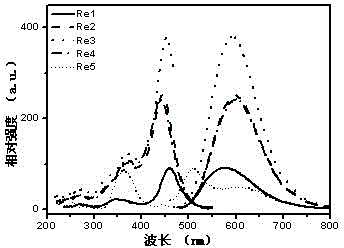
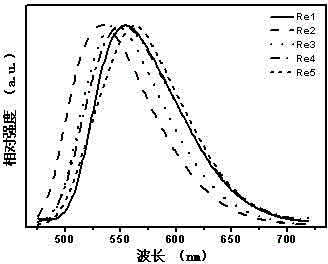
[0104] Chlorotricarbonyl (4-carbazole-2,2',4,2'-terpyridine) rhenium(I)(Re1)
[0105] l abs , nm 224, 277, 295, 397 (see figure 1 )
[0106] l ex , max , nm 459, 578 (see figure 2 )
[0107] l em , max , nm 551 (see image 3 )
[010...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com