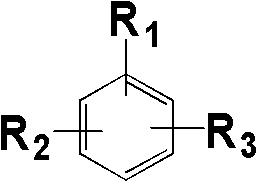
Phthalonitrile monomer containing aromatic polyamide, synthetizing method thereof and poly phthalonitrile resin produced through solidification of phthalonitrile monomer
A technology of polyphthalonitrile resin and phthalonitrile, which is applied to the preparation of carboxylic acid nitrile, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problem of increasing the complexity of resin processing and the large difference in molecular structure , unfavorable industrial production and other issues, to achieve the effect of avoiding complex blending process, simple method and easy promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
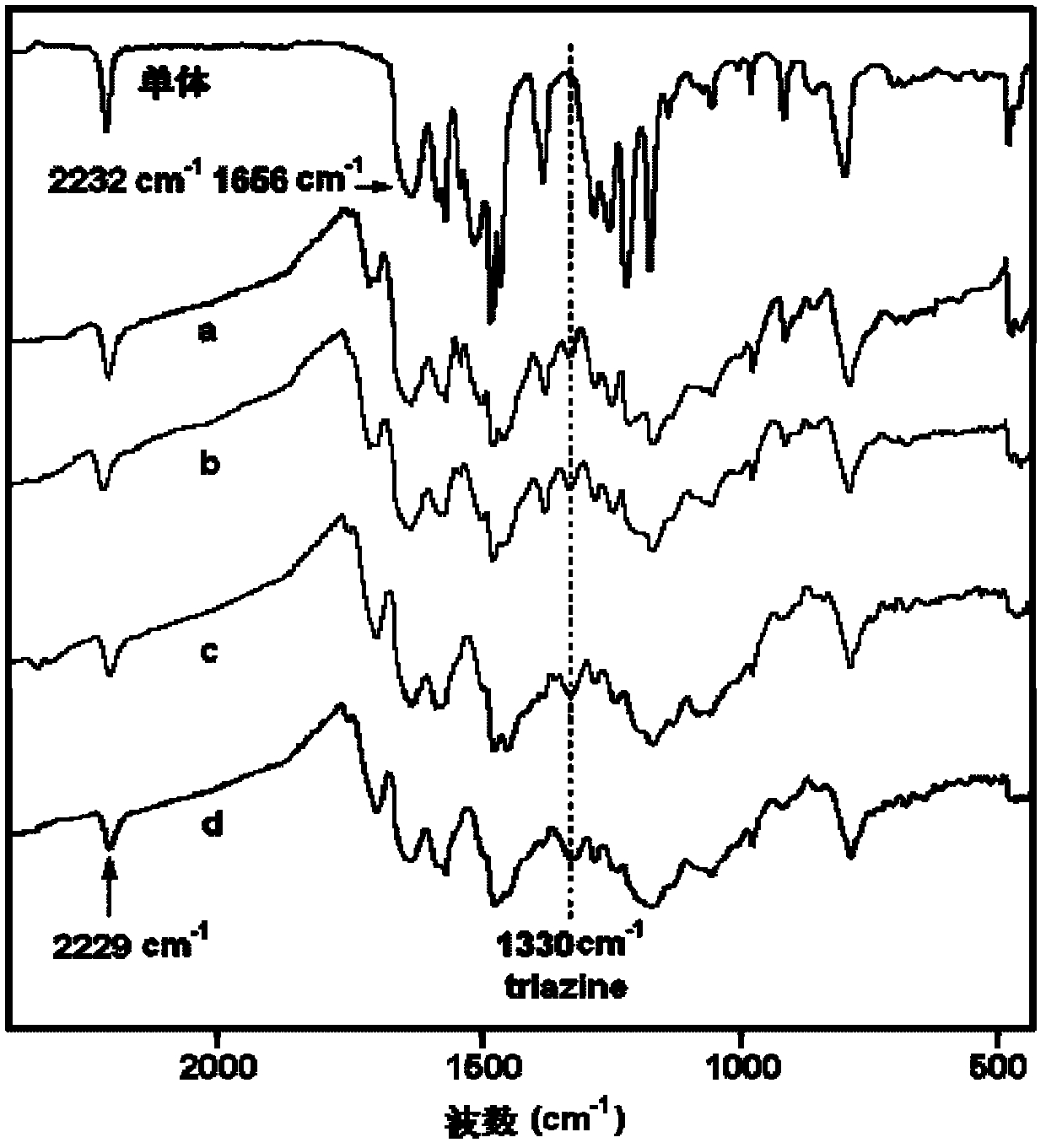
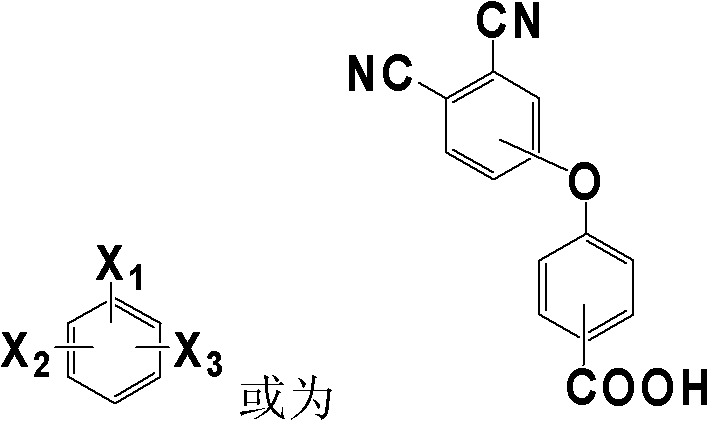
[0032] Add 4-(4-carboxyphenoxy)phthalonitrile and m-phenylenediamine in a molar ratio of 2:1 to DMF, stir and mix at room temperature, and make the solid content 30% (w / v), then add The dehydrating agent DCC is 1:2.5 based on the molar ratio of carboxylic acid raw materials, and reacted at 10°C for 12h. After the reaction, the filtrate was collected by filtration, and the filtrate was distilled to a solid content of 80% (w / v), and then the filter cake was collected by filtration. After the filter cake was dried at 80°C to constant weight, the phthalophthalamide containing aromatic amide with the following structure was obtained. Carbonitrile monomer 1. The minimum melt viscosity of the monomer 1 is 0.12Pa.S; IR(KBr, cm -1 ): 3315(N-H), 2231(C≡N), 1655(C=O), 1088(C-O-C); 1 H-NMR (DMSO-d6): 10.05 (2H, N-H), 8.02 (1H, Ar-H), 7.91 (4H, Ar-H), 7.65 (2H, Ar-H), 7.38 (4H, Ar-H ), 7.35(2H, Ar-H), 7.22(1H, Ar-H), 7.10(4H, Ar-H).
[0033] Put the above monomer 1 in a nitrogen atmosp...
Embodiment 2
[0035] Add 3-(4-carboxyphenoxy)phthalonitrile and p-phenylenediamine in a molar ratio of 2.1:1 into DMAc and stir at room temperature to mix evenly, and make the solid content 50% (w / v), then add The dehydrating agent TPP is 1:3 based on the molar ratio of the carboxylic acid raw material, and reacted at 80° C. for 4 hours. After the reaction, the filtrate was collected by filtration, and the filtrate was distilled to a solid content of 85% (w / v), and then the filter cake was collected by filtration. After the filter cake was dried at 90°C to a constant weight, the phthalophthalamide containing aromatic amide with the following structure was obtained. Carbonitrile monomer 2. The minimum melt viscosity of the monomer 2 is 0.32Pa.S; IR(KBr, cm -1 ): 3300(N-H), 2232(C≡N), 1658(C=O), 1091(C-O-C); 1H-NMR (DMSO-d6): 10.1 (2H, N-H), 7.91 (4H, Ar-H), 7.68 (2H, Ar-H), 7.62 (4H, Ar-H), 7.41 (2H, Ar-H ), 7.38(2H, Ar-H), 7.10(4H, Ar-H).
[0036] Put the above monomer 2 in a nitrogen a...
Embodiment 3
[0038] Add 4-(3-carboxyphenoxy)phthalonitrile and 1,3,5-m-phenylenediamine in a molar ratio of 3.5:1 into NMP, stir and mix evenly at room temperature, and make the solid content 20% (w / v), then add the dehydrating agent NNPB with a molar ratio of carboxylic acid raw materials of 1:5, and react at 30°C for 6h. After the reaction, the filtrate was collected by filtration, and the filtrate was distilled to a solid content of 90% (w / v), and then the filter cake was collected by filtration. After the filter cake was dried at 120°C to constant weight, the phthalamide containing aromatic amide of the following structure was obtained: Carbonitrile monomer 3. The minimum melt viscosity of the monomer 3 is 0.32Pa.S; IR(KBr, cm -1 ): 3308(N-H), 2230(C≡N), 1654(C=O), 1090(C-O-C);
[0039] 1 H-NMR (DMSO-d6): 10.08 (3H, N-H), 7.76 (3H, Ar-H), 7.67 (3H, Ar-H), 7.65 (3H, Ar-H), 7.61 (3H, Ar-H ), 7.40(3H, Ar-H), 7.38(3H, Ar-H), 7.35(3H, Ar-H), 7.17(3H, Ar-H).
[0040] Put the above-ment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com