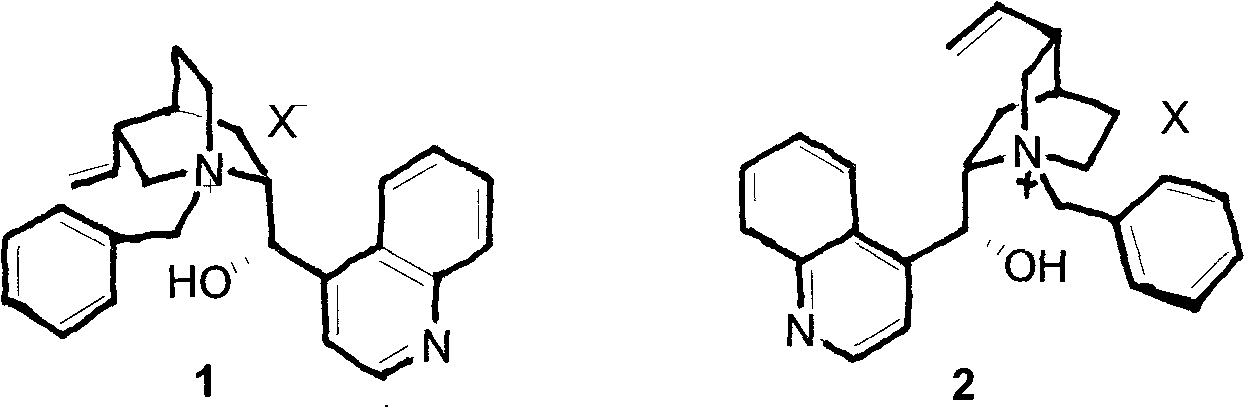
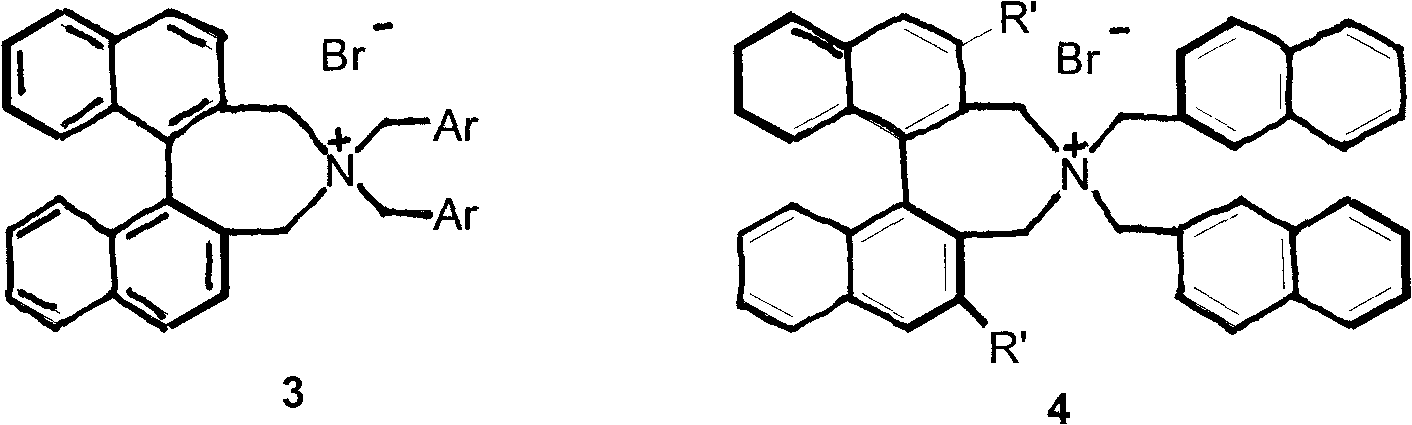
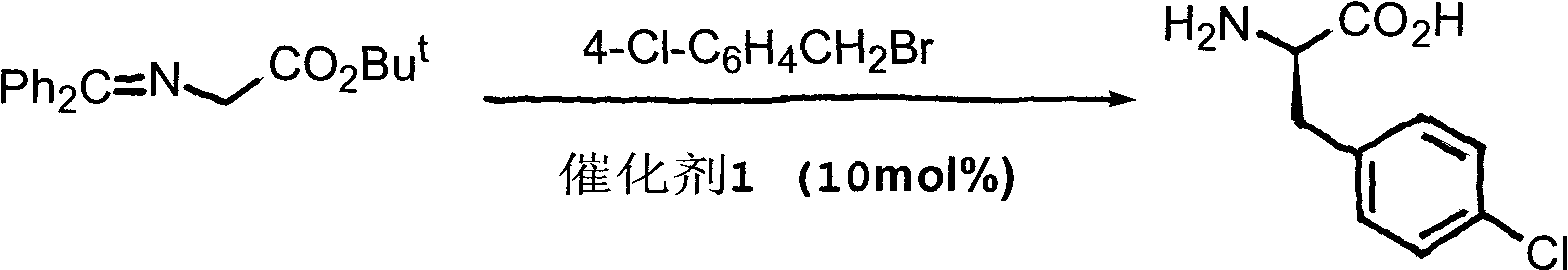Novel asymmetric phase-transfer catalyst pentaazabicyclo and preparation method thereof
A phase transfer catalyst, asymmetric technology, applied in chemical instruments and methods, preparation of imino compounds, catalysts for physical/chemical processes, etc., can solve problems such as inability to widely use organic synthesis reactions, insufficient catalyst activity, and large catalyst dosage. , to achieve the effect of a wide range of industrial applications, a wide range of applications, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The following will clearly and completely describe the technical solutions in the embodiments of the present invention in conjunction with the appended tables in the embodiments of the present invention. Obviously, the described embodiments are only some of the embodiments of the present invention, not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without creative efforts fall within the protection scope of the present invention.
[0030] The present invention provides a kind of synthetic route of novel asymmetric phase transfer catalyst pentaazabicyclic as follows:
[0031]
[0032] Reaction condition: step (i) adds triphosgene or thiophosgene, triethylamine; Step (ii) adds RX, NaH; Step (iii) adds oxalyl chloride, refluxes; Step (iv) adds ammonia, refluxes; Step ( v) add triethylamine and the product of step iii.
[0033] Specifically, Embodiment 1 of the present invention pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com